Advertisements
Advertisements
Question
What is meant by chemical reaction? Explain with the help of an example.
Solution
Chemical reaction is the process in which atoms of some elements rearrange—that is, undergo a chemical change—to form a new product with different properties. This involves breaking of old bonds between the reacting atoms and making of new chemical bonds between the rearranged atoms.
For example, the burning of magnesium ribbon in air. In this reaction, magnesium ribbon, on heating, combines with oxygen to form a white powder of magnesium oxide, which is totally different in its properties from magnesium and oxygen.
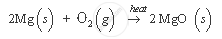
APPEARS IN
RELATED QUESTIONS
Is Burning of natural gas an endothermic reaction or an exothermic reaction?
A white precipitate can be obtained by adding dilute sulphuric acid to:
(a) CuSO4 solution
(b) Nacl solution
(c) BaCl2 solution
(d) Na2SO4 solution
What information do you get from the equation H2+ Cl2 → 2HCl ?
How is a flame test performed?
Define: Endothermic reaction
When a potassium iodide solution is added to a solution of lead (II) nitrate in a test tube, a precipitate is formed.
Write the balanced chemical equation for this reaction.
Balance the following simple equation:
H2 + O2 → H2O
Balance the following simple equation:
NaOH + Cl2 → NaCl + NaClO + H2O
In the chemical equation the _______ are written on the left-hand side.
Write three steps of writing chemical equations with example?
