Advertisements
Advertisements
प्रश्न
What is meant by chemical reaction? Explain with the help of an example.
उत्तर
Chemical reaction is the process in which atoms of some elements rearrange—that is, undergo a chemical change—to form a new product with different properties. This involves breaking of old bonds between the reacting atoms and making of new chemical bonds between the rearranged atoms.
For example, the burning of magnesium ribbon in air. In this reaction, magnesium ribbon, on heating, combines with oxygen to form a white powder of magnesium oxide, which is totally different in its properties from magnesium and oxygen.
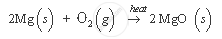
APPEARS IN
संबंधित प्रश्न
Why should a magnesium ribbon be cleaned before it is burnt in air?
What is a balanced chemical equation? Why should a chemical equation be balanced?
Balance the given equation:
HNO3 + Ca(OH)2  Ca(NO3)2 + H2O
Ca(NO3)2 + H2O
Write your observation for the following chemical reaction and name the product formed:
When sugar is heated.
Balance the equation stepwise.
Ag(s) + HCl(aq) → AgCl ↓+ H2 ↑
What information do the following chemical equations convey? Mg + 2HCl → MgCl2+ H2
Write the balanced chemical equation of the following reaction.
zinc sulphide + oxygen → zinc oxide + sulphur dioxide
Write the balanced chemical equation of the following reaction.
aluminium sulphate + sodium hydroxide → sodium sulphate + sodium meta aluminate + water.
\[\ce{2KClO3->[MnO2] 2KCl + 3O2[g] - is a balanced equation.}\]
State why the compound MnO2 is written above the arrow.
With reference to a chemical equation state which of the statements 1 to 5 pertain to A or B.
A: Information provided by a chemical equation.
B: Limitations of a chemical equation.
- The nature of the individual elements.
- The speed of the reaction.
- The state of matter in which the substance is present.
- The completion of the reaction.
- The direction of the reaction.
