Advertisements
Advertisements
प्रश्न
What is meant by chemical reaction? Explain with the help of an example.
उत्तर
Chemical reaction is the process in which atoms of some elements rearrange—that is, undergo a chemical change—to form a new product with different properties. This involves breaking of old bonds between the reacting atoms and making of new chemical bonds between the rearranged atoms.
For example, the burning of magnesium ribbon in air. In this reaction, magnesium ribbon, on heating, combines with oxygen to form a white powder of magnesium oxide, which is totally different in its properties from magnesium and oxygen.
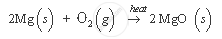
APPEARS IN
संबंधित प्रश्न
On what basis is a chemical equation balanced?
Which of the following is not an endothermic reaction?
(a) CaCO3 → CaO + CO2
(b) 2H2O →2H2 + O2
(c) 6CO2 + 6H2O → C6H12O6 + 6O2
(d) C6H12O6 + 6O2 → 6CO2 + 6H2O
Balance the following chemical equation :
FeS + HCl → FeCl2 + H2S
What is the limitation of the reaction given in question 2?
Write the balanced chemical equation of the following reaction. sodium hydroxide + sulphuric acid → sodium sulphate + water
Write the balanced chemical equation of the following reaction. iron + sulphuric acid → ferrous sulphate + hydrogen.
In certain reaction a change of state is observed i.e. solid to liquid, liquid to gas etc. – State the change of state of the products – to give the respective reactant.
NH3 + HCl ⇌ NH4Cl
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Iron [III] chloride to ammonium hydroxide.
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
Write the word equation for the above molecular equation.
Balance the following simple equation:
SO2 + O2 ⇌ SO3
