Advertisements
Advertisements
प्रश्न
When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products.
उत्तर

APPEARS IN
संबंधित प्रश्न
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are metals.
What do you understand by exothermic reactions?
Which of the following is not an endothermic reaction?
(a) CaCO3 → CaO + CO2
(b) 2H2O →2H2 + O2
(c) 6CO2 + 6H2O → C6H12O6 + 6O2
(d) C6H12O6 + 6O2 → 6CO2 + 6H2O
Balance the following chemical equation:
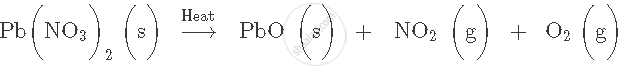
Write word equation for the following skeletal equation:
\[\ce{Zn + HCl -> ZnCl2 + H2}\]
Balance the following equation:
S + H2SO4 → SO2 + H2O
\[\ce{2KClO3->[MnO2] 2KCl + 3O2[g] - is a balanced equation.}\]
Give a reason why the above equation is balanced.
Which of the following are exothermic processes?
- Reaction of water with quick lime
- Dilution of an acid
- Evaporation of water
- Sublimation of camphor (crystals)
What are the steps involved in writing the skeletal equation?
A student took a small amount of copper oxide in a conical flask and added dilute hydrochloric acid to it with constant stirring. He observed a change in colour of the solution.
- Write the name of the compound formed and its colour.
- Write a balanced chemical equation for the reaction involved.
