Advertisements
Advertisements
Question
What are the various ways in which a chemical equation can be made more informative? Give examples to illustrate your answer.
Solution
A chemical equation can be made more informative by:
- Indicating physical states of reactants and products.
- Indicating heat change in the reaction.
- Indicating the reaction condition.
Example:
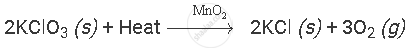
where,
(s) and (g) signifies solid and gaseous state of compounds, respectively.
Heat written on reactants' side signifies that the reaction consumes heat.
MnO2 written above right-handed arrow signifies reaction takes place in the presence of MnO2.
APPEARS IN
RELATED QUESTIONS
Translate the following statement into chemical equation and then balance it.
Hydrogen gas combines with nitrogen to form ammonia
Complete and balance the following equation:
CA (OH)2 + .............  CaCO3 + H2O
CaCO3 + H2O
Substitute formulae for names and balance the following equation:
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas.
Express the following facts in the form of a balanced chemical equation:
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
Write word equation for the following skeletal equation:
\[\ce{KNO3 -> KNO2 + O2}\]
Write the chemical equation for the following word equation and balance them.
Magnesium + Sulphuric acid → Magnesium sulphate + Hydrogen
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Copper [II] sulphate to sodium hydroxide.
Balance the following simple equation:
Al + O2 → Al2O3
Balance the following simple equation:
FeCl2 + Cl2 → FeCl3
To balance the following chemical equation the value of x and y should respectively be:
\[\ce{2NaOH + xAl_2O_3->yNaAlO_2 + H_2O}\]
