Advertisements
Advertisements
Question
Carbon monoxide reacts with hydrogen under certain conditions to form methanol (CH3OH). Write a balanced chemical equation for this reaction indicating the physical states of reactants and product as well as the conditions under which this reaction takes place.
Solution
Carbon monoxide reacts with hydrogen under the following conditions:
(i) 300 atm. pressure
(ii) 300oC temperature
(iii) in the presence of ZnO and CrO3 as catalysts
The balanced chemical equation for the given reaction is as follows:
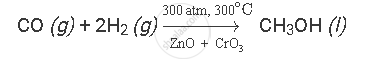
APPEARS IN
RELATED QUESTIONS
Write the balanced chemical equation for the following reaction.
\[\ce{Zinc + Silver nitrate -> Zinc nitrate + Silver}\]
State one characteristic of the chemical reaction which takes place when lemon juice is added gradually to potassium permanganate solution.
One of the following is an exothermic reaction. This is:
(a) electrolysis of water
(b) conversion of limestone into quicklime
(c) process of respiration
(d) process of photosynthesis
Balance the following equation :
Pb3O4 → PbO + O2
In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Iron [III] chloride to ammonium hydroxide.
What is meant by ‘reactants’ and ‘products’ in a chemical equation?
Balance the following simple equation:
Zn + NaOH → Na2ZnO2 + H2
Balance the following simple equation:
NaHCO3 + H2SO4 → Na2SO4 + H2O + CO2
Which of the following statements about the given reaction are correct?
`3"Fe"("s") + 4"H"_2"O"("g") -> "Fe"_3"O"_4("s") + 4"H"_2("g")`
- Iron metal is getting oxidised
- Water is getting reduced
- Water is acting as reducing agent
- Water is acting as oxidising agent
