Advertisements
Advertisements
Question
Translate the following statement into chemical equation and then balance it.
Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
Solution
\[\ce{BaCl2_{(aq)} + Al2(SO4)3_{(aq)} -> AlCl3_{(aq)} + BaSO4_{(s)}}\]
Balanced equation:
\[\ce{3BaCl2_{(aq)} + Al2(SO4)3_{(aq)} -> 2AlCl3_{(aq)} + 3BaSO4_{(s)}}\]
APPEARS IN
RELATED QUESTIONS
Fill in the following blank with suitable word:
Chemical equations are balanced to satisfy the law of ............
Write balanced chemical equation from the following information:
An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
Give one example of an endothermic reaction.
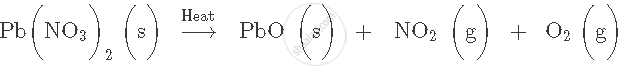
Balance the following chemical equation:
Write word equation for the following skeletal equation:
\[\ce{CO + O2 -> CO2}\]
Write word equation for the following skeletal equation:
\[\ce{NaOH + H2SO4 -> Na2SO4 + H2O}\]
Write word equation for the following skeletal equation:
\[\ce{KNO3 -> KNO2 + O2}\]
Write your observation for the following chemical reaction and name the product formed :
When an aqueous solution of sodium chloride is mixed with an aqueous solution of silver nitrate.
Write symbolic representation for the following word equation and balance them :
Aluminium + Chlorine → Aluminium chloride.
In the experiment for determining the percentage of water absorbed by raisins, we do the final weighing of the raisins after keeping them dipped in water for about one hour. For the accuracy of the result, the extra water from the surface of the soaked raisins is removed by
Balance the following equation:
P + HNO3 → NO2 + H2O + H3PO4
Write the balanced chemical equation of the following reaction. potassium bicarbonate + sulphuric acid → potassium sulphate + carbon dioxide + water
Write the balanced chemical equation of the following reaction.
aluminium carbide + water → aluminium hydroxide + methane
Write the balanced chemical equation of the following reaction.
aluminium sulphate + sodium hydroxide → sodium sulphate + sodium meta aluminate + water.
Grills of doors and windows are always painted before they are used.
Balance the following simple equation:
Zn + NaOH → Na2ZnO2 + H2
With reference to a chemical equation state which of the statements 1 to 5 pertain to A or B.
A: Information provided by a chemical equation.
B: Limitations of a chemical equation.
- The nature of the individual elements.
- The speed of the reaction.
- The state of matter in which the substance is present.
- The completion of the reaction.
- The direction of the reaction.
Write three steps of writing chemical equations with example?
A student took a small amount of copper oxide in a conical flask and added dilute hydrochloric acid to it with constant stirring. He observed a change in colour of the solution.
- Write the name of the compound formed and its colour.
- Write a balanced chemical equation for the reaction involved.
