Advertisements
Online Mock Tests
Chapters
2: Acids, Bases and Salts
3: Metals and Non-metals
4: Carbon and its Compounds
5: Life Processes
6: Control and Coordination
7: How do Organisms Reproduce?
8: Heredity
9: Light – Reflection and Refraction
10: The Human Eye and the Colourful World
11: Electricity
12: Magnetic Effects of Electric Current
13: Our Environment
![NCERT solutions for Science [English] Class 10 chapter 1 - Chemical Reactions and Equations NCERT solutions for Science [English] Class 10 chapter 1 - Chemical Reactions and Equations - Shaalaa.com](/images/science-english-class-10_6:1a59543cfe684fe9a4034b624a338d9a.jpg)
Advertisements
Solutions for Chapter 1: Chemical Reactions and Equations
Below listed, you can find solutions for Chapter 1 of CBSE, Karnataka Board NCERT for Science [English] Class 10.
NCERT solutions for Science [English] Class 10 1 Chemical Reactions and Equations EXERCISES [Pages 14 - 16]
Which of the statements about the reaction below are incorrect?
\[\ce{2PbO(s) + C(s) → 2Pb(s) + CO2(g)}\]
- Lead is getting reduced.
- Carbon dioxide is getting oxidised.
- Carbon is getting oxidised.
- Lead oxide is getting reduced.
(a) and (b)
(a) and (c)
(a), (b) and (c)
all
\[\ce{Fe2O3 + 2Al -> Al2O3 + 2Fe}\]
The above reaction is an example of a ______.
combination reaction
double displacement reaction
decomposition reaction
displacement reaction
What happens when dilute hydrochloric acid is added to iron fillings? Choose the correct answer.
Hydrogen gas and iron chloride are produced.
Chlorine gas and iron hydroxide are produced.
No reaction takes place.
Iron salt and water are produced.
What is a balanced chemical equation? Why should a chemical equation be balanced?
Translate the following statement into chemical equation and then balance it.
Hydrogen gas combines with nitrogen to form ammonia
Translate the following statement into chemical equation and then balance it.
Hydrogen sulphide gas burns in the air to give water and sulphur dioxide.
Translate the following statement into chemical equation and then balance it.
Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
Translate the following statement into chemical equation and then balance it.
Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
Balance the chemical equation.
\[\ce{HNO3 +Ca(OH)2 -> Ca(NO3)2 + H2O}\]
Balance the following chemical equation.
\[\ce{NaOH + H2SO4 -> Na2SO4 + H2O}\]
Balance the following chemical equation.
\[\ce{NaCl + AgNO3 -> AgCl + NaNO3}\]
Balance the following chemical equation.
\[\ce{BaCl2 + H2SO4 -> BaSO4 + HCl}\]
Write the balanced chemical equation for the following reaction.
\[\ce{Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water}\]
Write the balanced chemical equation for the following reaction.
\[\ce{Zinc + Silver nitrate -> Zinc nitrate + Silver}\]
Write the balanced chemical equation for the following reaction.
\[\ce{Aluminium + Copper chloride -> Aluminium chloride + Copper}\]
Write the balanced chemical equation for the following reaction.
\[\ce{Barium chloride + Potassium sulphate → Barium sulphate + Potassium chloride}\]
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Potassium bromide (aq) + Barium iodide (aq) -> Potassium iodide (aq) + Barium bromide (s)}\]
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Zinc carbonate(s) -> Zinc oxide(s) + Carbon dioxide(g)}\]
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Hydrogen(g) + Chlorine(g) -> Hydrogen chloride (g)}\]
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Magnesium(s) + Hydrochloric acid(aq) -> Magnesium chloride(aq) + Hydrogen(g)}\]
What does one mean by exothermic reaction? Give example.
What does one mean by endothermic reaction? Give example.
Why is respiration considered an exothermic reaction? Explain.
Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Write one equation for decomposition reactions where energy is supplied in the form of heat.
Write one equation for decomposition reactions where energy is supplied in the form of light.
Write one equation for decomposition reactions where energy is supplied in the form of electricity.
What is the difference between displacement and double displacement reactions? Write equations for these reactions.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
What do you mean by a precipitation reaction? Explain by giving examples.
Explain the following in term of gain or loss of oxygen with two examples.
Oxidation
Explain the following in term of gain or loss of oxygen with two examples.
Reduction
A shiny brown-coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black-colored compound formed.
Why do we apply paint on iron articles?
Oil and fat containing food items are flushed with nitrogen. Why?
Explain the terms Corrosion
Explain the term Rancidity.
NCERT solutions for Science [English] Class 10 1 Chemical Reactions and Equations Intext Questions [Pages 6 - 13]
Why should a magnesium ribbon be cleaned before it is burnt in air?
Write the balanced equation for the following chemical reaction.
\[\ce{Hydrogen + Chlorine -> Hydrogen chloride}\]
Write the balanced equation for the following chemical reaction.
\[\ce{Barium chloride + Aluminium sulphate -> Barium sulphate + Aluminium chloride}\]
Write the balanced equation for the following chemical reaction.
\[\ce{Sodium + Water -> Sodium hydroxide + Hydrogen}\]
Write a balanced chemical equation with state symbols for the following reaction.
Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
Write a balanced chemical equation with state symbols for the following reaction.
Sodium hydroxide solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
A solution of a substance ‘X’ is used for white washing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in (i) above with water.
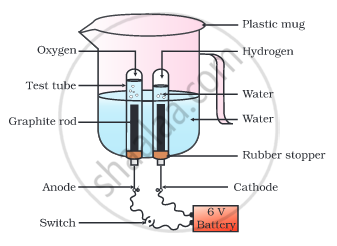
Why is the amount of gas collected in one of the test tubes in the following Activity double of the amount collected in the other? Name this gas.
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in the following Fig.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- Is the volume of the gas collected the same in both the test tubes?
• Once the test tubes are filled with the respective gases, remove them carefully. - Test these gases one by one by bringing a burning candle close to the mouth of the test tubes.
Caution: This step must be performed carefully by the teacher.
- What happens in each case?
- Which gas is present in each test tube?
Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Give an example of a double displacement reaction.
Identify the substances that are oxidised and the substances that are reduced in the following reaction.
\[\ce{4Na(s) + O2(g) -> 2Na2O(s)}\]
Identify the substances that are oxidised and the substances that are reduced in the following reaction.
\[\ce{CuO(s) + H2(g) -> Cu(s) + H2O (l)}\]
Solutions for 1: Chemical Reactions and Equations
![NCERT solutions for Science [English] Class 10 chapter 1 - Chemical Reactions and Equations NCERT solutions for Science [English] Class 10 chapter 1 - Chemical Reactions and Equations - Shaalaa.com](/images/science-english-class-10_6:1a59543cfe684fe9a4034b624a338d9a.jpg)
NCERT solutions for Science [English] Class 10 chapter 1 - Chemical Reactions and Equations
Shaalaa.com has the CBSE, Karnataka Board Mathematics Science [English] Class 10 CBSE, Karnataka Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. NCERT solutions for Mathematics Science [English] Class 10 CBSE, Karnataka Board 1 (Chemical Reactions and Equations) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. NCERT textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Science [English] Class 10 chapter 1 Chemical Reactions and Equations are Chemical Equation, Direct Combination (or Synthesis) Reaction, Decomposition Reactions, Types of Chemical Change or Chemical Reaction, Balancing Chemical Equation, Single Displacement Reactions, Double Displacement Reaction, Oxidation, Reduction and Redox Reactions, Corrosion of Metals, Rancidity of Food and Its Prevention, Chemical Equation, Direct Combination (or Synthesis) Reaction, Decomposition Reactions, Types of Chemical Change or Chemical Reaction, Balancing Chemical Equation, Single Displacement Reactions, Double Displacement Reaction, Oxidation, Reduction and Redox Reactions, Corrosion of Metals, Rancidity of Food and Its Prevention, Chemical Equation, Direct Combination (or Synthesis) Reaction, Decomposition Reactions, Types of Chemical Change or Chemical Reaction, Balancing Chemical Equation, Single Displacement Reactions, Double Displacement Reaction, Oxidation, Reduction and Redox Reactions, Corrosion of Metals, Rancidity of Food and Its Prevention.
Using NCERT Science [English] Class 10 solutions Chemical Reactions and Equations exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in NCERT Solutions are essential questions that can be asked in the final exam. Maximum CBSE, Karnataka Board Science [English] Class 10 students prefer NCERT Textbook Solutions to score more in exams.
Get the free view of Chapter 1, Chemical Reactions and Equations Science [English] Class 10 additional questions for Mathematics Science [English] Class 10 CBSE, Karnataka Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
