Advertisements
Advertisements
Question
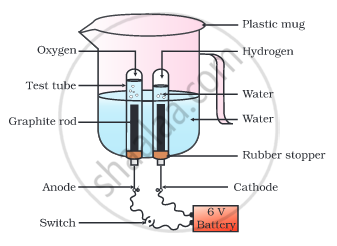
Why is the amount of gas collected in one of the test tubes in the following Activity double of the amount collected in the other? Name this gas.
- Take a plastic mug. Drill two holes at its base and fit rubber stoppers in these holes. Insert carbon electrodes in these rubber stoppers as shown in the following Fig.
- Connect these electrodes to a 6 volt battery.
- Fill the mug with water such that the electrodes are immersed. Add a few drops of dilute sulphuric acid to the water.
- Take two test tubes filled with water and invert them over the two carbon electrodes.
- Switch on the current and leave the apparatus undisturbed for some time.
- You will observe the formation of bubbles at both the electrodes. These bubbles displace water in the test tubes.
- Is the volume of the gas collected the same in both the test tubes?
• Once the test tubes are filled with the respective gases, remove them carefully. - Test these gases one by one by bringing a burning candle close to the mouth of the test tubes.
Caution: This step must be performed carefully by the teacher.
- What happens in each case?
- Which gas is present in each test tube?
Solution
Water contains two parts hydrogen and one part oxygen. Therefore, the amount of hydrogen and oxygen produced during the electrolysis of water is in the ratio of 2:1. In the process of electrolysis, hydrogen goes into one test tube, and oxygen goes into another. Therefore, the amount of hydrogen gas collected in the test tube is double that of oxygen.
APPEARS IN
RELATED QUESTIONS
Write one equation for decomposition reactions where energy is supplied in the form of heat.
Taking into consideration the relationship in the first pair, complete the second pair
2H2 + O2 → 2H2O :Combination Reaction :: 2HgO → 2Hg + O2:_________
State an important use of decomposition reactions.
What type of chemical reaction is used to extract metals from their naturally occurring compounds like oxides or chlorides?
What type of chemical reaction take place when silver bromide is exposed to sunlight?
What type of chemical reaction take place when electricity is passed through water?
State the change in colour observed in following case mentioning the reason:
Silver chloride is exposed to sunlight.
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
Two metals X and Y form the salts XSO4 and Y2SO4, respectively. The solution of salt XSO4 is blue in colour whereas that of Y2SO4 is colourless. When barium chloride solution is added to XSO4 solution, then a white precipitate Z is formed alongwith a salt which turns the solution green. And when barium chloride solution is added to Y2SO4 solution, then the same white precipitate Z is formed alongwith colourless common salt solution.
(a) What could the metals X and Y be?
(b) Write the name and formula of salt XSO4.
(c) Write the name and formula of salt Y2SO4.
(d) What is the name and formula of white precipitate Z?
(e) Write the name and formula of the salt which turns the solution green in the first case.
A student wants to study a decomposition reaction by taking ferrous sulphate crystals. Write two precautions he must observe while performing the experiment.
Give a balanced equation for –
A thermal decomposition reaction involving heat on limestone [calcium carbonate]
Give a balanced equation for –
An electrolytic decomposition reaction involving a neutral liquid
Give a balanced equation for –
A double decomposition neutralization reaction involving an acid and a base
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a metallic nitrate decomposes to give – a basic oxide.
Assertion: Decomposition of vegetable matter into compost is an endothermic reaction.
Reason: Decomposition reaction involves breakdown of a single reactant into simpler products.
Marble statues are corroded or stained rain water. Identify the main reason.
Balance the following chemical equation and identify the type of chemical reaction.
`"HgO"("s") overset("(heat)")(->) "Hg"("l") + "O"_2("g")`
Balance the following chemical equation and identify the type of chemical reaction.
`"H"_2"O"_2("l") overset("U V")(->) "H"_2"O"("l") + "O"_2("g")`
These consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Assertion: Silver bromide decomposition is used in black and white photography.
Reason: Light provides energy for this exothermic reaction.
