Advertisements
Advertisements
Question
Give a balanced equation for –
An electrolytic decomposition reaction involving a neutral liquid
Solution
Electrolytic decomposition of neutral liquid [water]
\[\ce{2H2O->[Electricity][Current]->2H2 + O2}\]
APPEARS IN
RELATED QUESTIONS
Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Write one equation for decomposition reactions where energy is supplied in the form of heat.
Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity
Give one example of a decomposition reaction which is carried out by applying heat.
What type of reaction is represented by the following equation?
CaCO3 → CaO + CO2
Identify the type of following reaction :
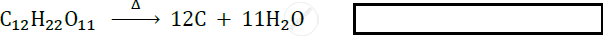
Complete the statement by filling in the blank with the correct word:
Decomposition of silver salts in the presence of sunlight is an example of _________.
Give the ratio in which hydrogen and oxygen are present in water by volume.
When lead nitrate is heated strongly in a boiling tube, two gases are liberated and a solid residue is left behind in the test tube.
- Name the type of chemical reaction and define it.
- Write the name and formula of the coloured gas liberated.
- Write the balanced chemical equation for the reaction.
- Name the residue left in the test tube and state the method of testing its nature (acidic/basic).
A solution of a substance ‘X’ is used for whitewashing.
- Name the substance ‘X’ and write its formula.
- Write the reaction of the substance ‘X’ named in the above formula with water.
