Advertisements
Advertisements
Question
Options
Fe + S ⟶ FeS.
HCl + NaOH ⟶ NaCl + H2O
CuSO4 + Zn ⟶ ZnSO4 + Cu
\[\ce{CaCO3 ->[\Delta]CaO + CO2}\]
Solution
\[\ce{CaCO3 ->[\Delta]CaO + CO2}\] is a decomposition reaction.
APPEARS IN
RELATED QUESTIONS
Write one equation for decomposition reactions where energy is supplied in the form of heat.
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P.
(a) What is substance P? Write its common name as well as chemical formula.
(b) What is substance Q?
(c) What is gas R?
(d) What is substance S? What is its clear solution known as?
(e) What is substance T? Name any two natural forms in which substance T occurs in nature.
State an important use of decomposition reactions.
Give one example of a decomposition reaction which is carried out by applying heat.
Give one example of a decomposition reaction which is carried out with electricity.
What type of chemical reaction is used to extract metals from their naturally occurring compounds like oxides or chlorides?
What type of chemical reaction take place when lime-stone is heated?
What type of chemical reaction take place when silver bromide is exposed to sunlight?
What is the colour of ferrous sulphate crystals? How does this colour change after heating?
Name the product formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Which of the following can be decomposed by the action of light?
(a) NaCl
(b) KCl
(c) AgCl
(d) CuCl
When a green iron salt is heated strongly, its colour finally changes to brown and odour of burning sulphur is given out.
(a) Name the iron salt.
(b) Name the type of reaction that takes place during the heating of iron salt.
(c) Write a chemical equation for the reaction involved.
Classify the following reaction as combination, decomposition, displacement, precipitation and neutralization. Also balance the equation.
\[\ce{Zn_{(s)} + H2SO4 -> ZnSO4_{(s)} + H2_{(g)}}\]
What are thermal decomposition reactions ? Explain with an example.
What do you mean by redox reaction ? Explain with the help of an example.
Identify the type of following reaction :
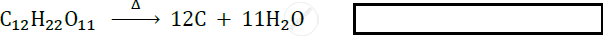
Differentiate between direct combination reaction and a decomposition reaction.
Give a balanced equation for –
A thermal decomposition reaction involving heat on limestone [calcium carbonate]
Give a balanced equation for –
A double decomposition neutralization reaction involving an acid and a base
Give a balanced equation for –
A white precipitate obtained during a double decomposition reaction involving a silver salt with sodium salt.
Give a balanced equation for the following type of reaction:
A thermal decomposition reaction in which a metallic nitrate decomposes to give – a basic oxide.
What is electrolysis?
Explain the reaction given in the figure.

The following reaction is used for the preparation of oxygen gas in the laboratory:
\[\ce{2KClO3_{(s)} ->[Heat] 2KCl + 3O2_{(g)}}\]
Which of the following statement about the reaction is correct?
Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
Which one of the following processes involves chemical reactions?
Which of the following is an endothermic process?
On heating blue coloured powder of copper (II) nitrate, in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed
- Write a balanced chemical equation of the reaction.
- Identify the brown gas X evolved.
- Identify the type of reaction.
- What could be the pH range of aqueous solution of the gas X?

- Identify the gasses evolved at the anode and cathode in the above experimental set up.
- Name the process that occurs. Why is it called so?
- Illustrate the reaction of the process with the help of a chemical equation.
Complete the following reaction:
\[\ce{C_12H_22O11->[Heat]}\] ______ + ______.
Write the molecular formula of calcium carbonate.
