Advertisements
Advertisements
Questions
Explain the terms Corrosion
Explain the term "corrosion" with an example.
Solution 1
Corrosion is defined as a process where materials, usually metals, deteriorate as a result of a chemical reaction with air, moisture, chemicals, etc. For example, in the presence of moisture, iron reacts with oxygen to form hydrated iron oxide.
\[\ce{4Fe + 3O2 + H2O → 2Fe2O3.nH2O}\]
Solution 2
Corrosion is a process where a refined metal is oxidised by atmospheric oxygen to form a more stable compound, such as oxides. The metal gradually degrades during the corrosion process. Iron rusting is a good example of corrosion, where the iron is converted to iron oxide. Millions of dollars are spent annually to prevent rusting on bridges and other monuments.
Solution 3
Corrosion is the process by which metal corrodes when it comes into contact with its surroundings, such as moisture, air, and acids. For example, a powdered (brown) coating on iron objects such as iron railings.
APPEARS IN
RELATED QUESTIONS
Give two examples of alloys with their chemical composition.
Tinning : Tin : : Galvanizing : _________
Which metals do not corrode easily?
Two methods by which rusting of iron can be prevented are ______ and ______.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What special name is given to their corrosion of iron?
What type of chemical reaction is involved in the corrosion of iron?
What is meant by galvanisation? Why is it done?
Explain why, iron sheets are coated with zinc.
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
What are the constituents of stainless steel?
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Bronze is an alloy of ______.
State under what conditions corrosion is faster ?
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
Choose the correct alternative and rewrite the following:
Iron is _____________________.
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Graphite is a lustrous non-metal which conducts electricity.
Corrosion of silver causes a black layer of _______.
To prevent corrosion of iron and steel _______ method is used.
_______ forms a green colour in the water.
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Write scientific reason.
Coins are made from metals and alloys.
Complete flow chart given below.

Observe the following diagram and give answers.
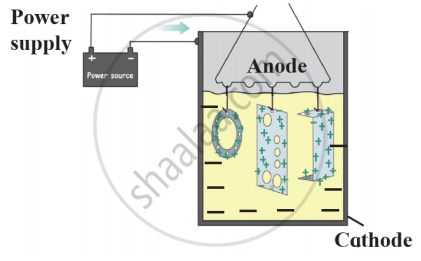
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Give the equation for the formation of rust.
State two conditions necessary for rusting of iron.
Which among the following alloys contain mercury as one of its constituents?
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
Galvanisation is a process used to prevent the rusting of which the following?
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
Give an example of a chemical reaction for each of the following situations:
Sound is produced
