Advertisements
Advertisements
Question
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
Solution
Calcination
APPEARS IN
RELATED QUESTIONS
Why do we apply paint on iron articles?
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Name five methods of preventing rusting of iron.
Explain why, the galvanised iron article is protected against rusting even if the zinc layer is broken.
Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Write three methods of preventing rusting of iron.
Bronze : _______ : : Stainless steel : Fe + Cr + C
The diagram shows the reaction between metal and dil. acid.
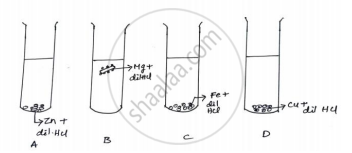
What is the reason for different behaviour of Mg in test tube B?
Galvanisation is a process used to prevent the rusting of which the following?
Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
