Advertisements
Advertisements
Question
Explain why, the galvanised iron article is protected against rusting even if the zinc layer is broken.
Solution
The galvanised iron-zinc oxide layer coating on iron objects prevents zinc and iron under it from corrosion. This happens because zinc is more reactive than iron. If the zinc layer is broken, zinc, being more reactive, gets oxidised before iron and prevents its corrosion.
APPEARS IN
RELATED QUESTIONS
Explain how painting of an iron gate prevents it from rusting.
Write a chemical equation to show the process of corrosion of iron.
What special name is given to their corrosion of iron?
State two conditions for the rusting of iron.
Name five methods of preventing rusting of iron.
Name the metal which is a constituent of plant pigment?
Explain how the activity series accounts for each of the following:
tendency to corrosion
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
Bronze : _______ : : Stainless steel : Fe + Cr + C
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Write the name.
An alloy of copper and tin-
Write a molecular formula for rust.
What is rust?
Galvanisation is a method of protecting iron from rusting by coating it with a thin layer of ____________.
The diagram shows the reaction between metal and dil. acid.
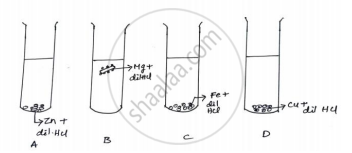
What is the reason for different behaviour of Mg in test tube B?
A man painted his main gate made up of iron, to
- prevent it from rusting.
- protect it from the sun.
- make it look beautiful.
- make it dust-free.
Which of the above statement(s) is/are correct?
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
Galvanisation is a process used to prevent the rusting of which the following?
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
