Advertisements
Advertisements
Question
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Solution
| Group A | Group B |
| 1) Electroplating | b) Silver plated spoons |
| 2) Anodising | a) Pressure cooker |
APPEARS IN
RELATED QUESTIONS
What is an alloy?
Why do we apply paint on iron articles?
Explain the terms Corrosion
Answer the following question.
What are alloys?
Explain why rusting of iron objects is faster in coastal areas than in deserts.
What is anodising? Give its applications.
What type of chemical reaction is involved in the corrosion of iron?
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
Name the metal which is used for galvanising iron.
Fill in the following blank with suitable word:
The corrosion of iron is called ................
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Name any two metals which do not corrode easily.
What are the constituents of stainless steel?
What are the special properties of stainless steel?
Name an alloy of copper. State its chemical composition and any one use.
Explain why, the galvanised iron article is protected against rusting even if the zinc layer is broken.
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Four metals P, Q, R and S are all obtained by the reduction of their oxides with carbon. Metal P is used to form a thin layer over the sheets of metal S to prevent its corrosion. Metal Q is used for electroplating tiffin boxes made of metal S whereas metal R is used in making car batteries. Metals Q and R form an alloy called solder. What are metals P, Q, R and S? How have you arrived at this conclusion?
Bronze is an alloy of ______.
State under what conditions corrosion is faster ?
Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
Corrosion of silver causes a black layer of _______.
_______ forms a green colour in the water.
Find the odd one out and give its explanation.
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Write scientific reason.
Coins are made from metals and alloys.
Explain concept with example/explain with the help of a balanced equation.
Corrosion
Draw a neat labelled diagram.
Anodizing
Write a molecular formula for rust.
Observe the following diagram and give answers.
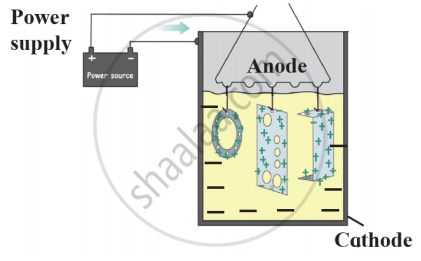
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Give the equation for the formation of rust.
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
Galvanisation is a process used to prevent the rusting of which the following?
Explain the following:
Lime water turns milky on passing carbon dioxide gas into it.
Give scientific reasons.
Silver amalgam is used for filling dental cavities.
Explain the chemical reactions in rusting of iron.
