Advertisements
Advertisements
Question
What type of chemical reaction is involved in the corrosion of iron?
Solution
Redox reaction is involved in the corrosion of iron.
APPEARS IN
RELATED QUESTIONS
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
What is meant by 'rusting of iron'? With the help of labelled diagrams, describe an activity to find out the conditions under which iron rusts.
Name an alloy of copper. State its chemical composition and any one use.
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
A metal X which is resistant to corrosion is produced by the electrolysis of its molten oxide whereas another metal Y which is also resistant to corrosion is produced by the reduction of its oxide with carbon. Metal X can be used in powder form in thermite welding whereas metal Y is used in making cathodes of ordinary dry cells.
(a) Name the metals X and Y.
(b) Which of the two metals is more reactive : X or Y?
(c) Name one ore or metal X. Also write its chemical formula.
(d) Name one ore or metal Y. Also write its chemical formula.
(e) Name one alloy of metal X and one alloy of metal Y.
Observe the following picture a write down the chemical reaction with the explanation.

Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Identify the process shown in the diagram and explain it in short
Bronze : _______ : : Stainless steel : Fe + Cr + C
Write scientific reason.
Anodization method is useful for prevention of the corrosion of the aluminium.
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Complete flow chart given below.

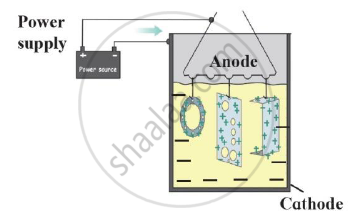
Observe the following diagram and give answers.
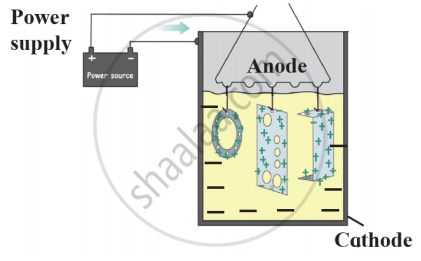
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
The process of coating the surface of the metal with a thin layer of zinc is called ______
Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is:
Galvanisation is a process used to prevent the rusting of which the following?
Explain the chemical reactions in rusting of iron.
| A process of forming a thick oxide of aluminium when aluminium is exposed to air. This coat makes it resistant to corrosion. Resistance can be improved by making a layer of oxide thinker. In this technique, the aluminium article is the anode, and the electrolyte is sulphuric acid. The anode reaction results in the formation of a black-coloured film of aluminium oxide on the anode. By putting appropriate dyes in the electrolytic solution, both coloured surface with the decorative finish is achieved. Kitchen articles like anodised such as pressure cookers, pans and frames of sliding windows are applications of this technique. |
- Name the anode and electrolyte used in this technique.
- How can we make aluminium articles made resistant to corrosion?
- Name the technique used to coat the aluminium articles.
