Advertisements
Advertisements
Question
Bronze : _______ : : Stainless steel : Fe + Cr + C
Solution
Bronze : Cu + Sn : : Stainless steel : Fe + Cr + C
APPEARS IN
RELATED QUESTIONS
Give two examples of alloys with their chemical composition.
Tinning : Tin : : Galvanizing : _________
Answer the following question.
What are alloys?
Two methods by which rusting of iron can be prevented are ______ and ______.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
Write a chemical equation to show the process of corrosion of iron.
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
Name five methods of preventing rusting of iron.
Explain why, the galvanised iron article is protected against rusting even if the zinc layer is broken.
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
No chemical reaction takes place when granules of a rusty-brown solid A are mixed with the powder of another solid B. However, when the mixture is heated, a reaction takes place between its components. One of the products C is a metal and settles down in the molten state while the other product D floats over it. It was observed that the reaction is highly exothermic.
(a) What could the solids A and B be?
(b) What are the products C and D most likely to be?
(c) Write the chemical equation for the reaction between A and B leading to the formation of C and D. Mention the physical states of all the reactants and products in this equation and indicate the heat change which takes place.
(d) What is the special name of such a reaction? State one use of such a reaction.
(e) Name any two types of chemical reactions under which the above reaction can be classified.
Explain how the activity series accounts for each of the following:
tendency to corrosion
Corrosion can be an advantage in some case.Explain ?
Compare roasting and calcination.
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
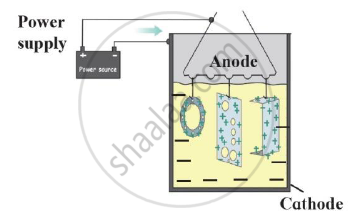
Identify the process shown in the diagram and explain it in short
Find the odd man out:
What are the adverse effects of corrosion?
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
What is "rusting"? Describe with a labelled diagram an activity to investigate the conditions under which iron rusts.
Give reason.
Copper and brass utensils should be tinned.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
Give a reason why rust turns moist red litmus blue.
Write a short note on Alloying.
When one of the metals in an alloy is mercury the alloy is called _______.
Find the odd one out and give its explanation.
Find the odd one out and give its explanation.
Write scientific reason.
Coins are made from metals and alloys.
Which among the following alloys contain mercury as one of its constituents?
Identify the correct statement from the following:
Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Explain the chemical reactions in rusting of iron.
