Advertisements
Advertisements
Question
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Solution
When acetic acid is added to a solution of sodium hydrogen carbonate, carbon dioxide gas is formed which comes out in the form of bubbles.
Sodium hydrogen carbonate + Acetic acid → Sodium carbonate + Water + Carbon dioxide
APPEARS IN
RELATED QUESTIONS
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Name any two metals which do not corrode easily.
What are the constituents of stainless steel?
State under what conditions corrosion is faster ?
Identify the process shown in the diagram and explain it in short
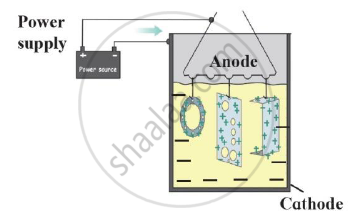
When one of the metals in an alloy is mercury the alloy is called _______.
Bronze : _______ : : Stainless steel : Fe + Cr + C
Observe the following diagram and give answers.
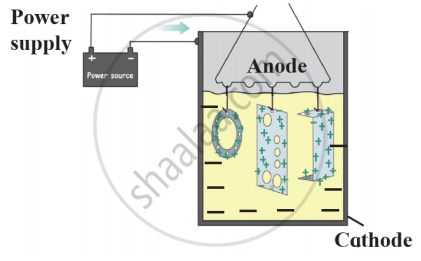
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Amalgam is an alloy of ____________.
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
