Advertisements
Advertisements
Question
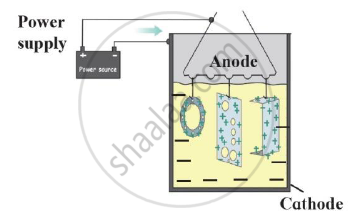
Identify the process shown in the diagram and explain it in short
Solution
The process shown in the diagram is Anodization.
In this method metals like copper, aluminium are coated with a thin and strong layer of their oxides by means of electrolysis. For this the copper or aluminium article is used as anode. As this oxide layer is strong and uniform all over the surface, it is useful for prevention of the corrosion of the metal.
APPEARS IN
RELATED QUESTIONS
What is an alloy?
Give two examples of alloys with their chemical composition.
Tinning : Tin : : Galvanizing : _________
Why do we apply paint on iron articles?
Which of the following methods is suitable for preventing an iron frying pan from rusting?
Two methods by which rusting of iron can be prevented are ______ and ______.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
What special name is given to their corrosion of iron?
Explain why, iron sheets are coated with zinc.
Why is an iron grill painted frequently?
Explain why, through aluminium is more reactive than iron, yet there is less corrosion of aluminium when both are exposed to air.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
Name five methods of preventing rusting of iron.
What are the constituents of stainless steel?
What are the special properties of stainless steel?
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
Name a common metal which is highly resistant to corrosion.
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Name the metal which is a constituent of blood pigment?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Observe the following picture a write down the chemical reaction with the explanation.

Write scientific reasons.
Lemon or tamarind is used for cleaning copper vessels turned greenish.
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
_______ is an alloy made from iron, carbon and chromium.
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
Bronze : _______ : : Stainless steel : Fe + Cr + C
Find the odd one out and give its explanation.
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Write scientific reason.
Anodization method is useful for prevention of the corrosion of the aluminium.
What is rust?
Write a molecular formula for rust.
Copper objects lose their shine and form green coating of ____________.
Which among the following alloys contain mercury as one of its constituents?
The diagram shows the reaction between metal and dil. acid.
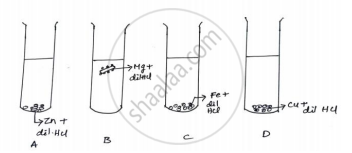
What is the reason for different behaviour of Mg in test tube B?
Gold plated ornaments is the example of ______.
In ______ process a layer of molten tin is deposited on metals.
