SSC (English Medium)
SSC (Marathi Semi-English)
Academic Year: 2018-2019
Date: March 2019
Advertisements
i) If g = GM/r2 then where will the value of g be high at Goa Beach or on top of Mount Everest?
Chapter: [0.01] Gravitation
Identify from the reaction the reactants that undergo oxidation and reduction.
Fe + S → FeS
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Find the odd one out and justify it.
Fuse wire, M.C.B., Rubber Gloves, Generator
Chapter: [0.04] Effects of Electric Current [0.14] The Electric Spark
Name the defect shown in the diagram.
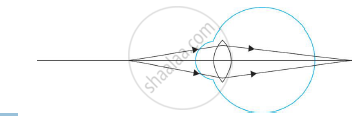
Chapter: [0.07] Lenses
Molecular formula of Propane is C3H8 , write the structural formula of propane.
Chapter:
The halogen which is liquid at room temperature is ..................
a) fluorine
b) astetine
c) bromine
d) iodine
Chapter: [0.02] Periodic Classification of Elements
Which of the following process to be carried out to avoid the
formation of greenish layer on brass vessels due to corrosion?
a) plating
b) anodization
c) tinning
d) alloying
Chapter: [0.08] Metallurgy
Advertisements
What type of reaction is shown below?

a) Addition
b) Substitution
c) Decomposition
d) Reduction
Chapter:
The temperature of ice can be decreased below 0°C by mixing _______ in it.
Saw dust
Sand
Salt
Coal
Chapter:
The image obtained while finding the focal length of convex lens is ....................
a) a real and erect.
b) virtual and erect.
c) real and inverted.
d) virtual and inverted.
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
i) Observe the following reaction and answer the following questions.
`CuSO_4 (aq) + Fe (s) → FeSO_4(aq) + Cu (s)`
a) Identify and write the type of chemical reaction.
b) Write the definition of above reaction.
Chapter: [0.03] Chemical Reactions and Equations [0.12] The Magic of Chemical Reactions
Light travels with a velocity 1.5 x 108 m/s in a medium. On entering second medium its velocity becomes 0.75 x 108 m/s. What is the refractive index of the second medium with respect to the first medium?
Chapter: [0.06] Refraction of Light [0.17] Wonders of Light 2
iii) Observe the figure and answer the following questions.
a) Identify the block shown by box A and write an electronic configuration of any one element of this block.
b) Identify the block of element denoted by letter B and write its period number.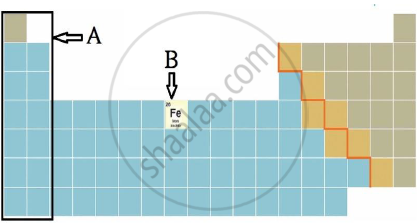
Chapter: [0.02] Periodic Classification of Elements
Write the IUPAC names of following hydrocarbons.
a) CH3-CHOH-CH3
b) CH3-CH2-COOH
Chapter:
Why does tungsten metal used to make solenoid type coil in an electric bulb?
Chapter: [0.04] Effects of Electric Current [0.14] The Electric Spark
Mahendra and Virat are sitting at a distance of 1 metre from each other. Their masses are 75 kg and 80 kg respectively. What is the gravitational force between them? G = 6.67 x 10-11 Nm2/kg2
Chapter: [0.1] Space Missions
Advertisements
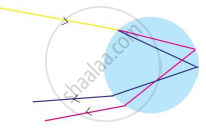
Identify the process shown in the diagram and explain it in short
Chapter: [0.03] Chemical Reactions and Equations [0.08] Metallurgy
i) Observe the given figure and answer the following questions.
a) Identify and write the natural process shown in the figure.
b) List the phenomena which are observe in this process.
c) Redraw the diagram and show above phenomena in it.
Chapter:
Identify the law shown in the figure and state three respective laws.
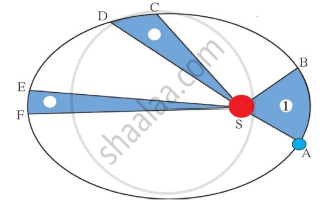
Chapter: [0.01] Gravitation
An element has its electron configuration as 2,8,8,2. Now answer the following questions.
a) What is the atomic number of this element?
b) What is the group of this element?
c) To which period does this element belong?
Chapter: [0.08] Metallurgy
Write the importance of artificial satellites in your words.
Chapter: [0.1] Space Missions
Observe the figure and answer the following questions.
a) Identify the machine shown in figure.
b) Write a use of this machine.
c) How transformation of energy takes place in this machine.
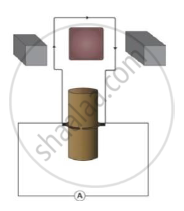
Chapter: [0.15] All about Electromagnetism
Balance the following equation stepwise.
\[\ce{NaOH + H2SO4 -> Na2SO4 + H2O}\]
Chapter: [0.03] Chemical Reactions and Equations
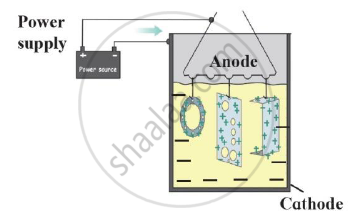
Identify the process given in following passage and draw neat labelled diagram showing the process.
Electrolysis of molten mixture of alumina (melting point > 20000C) is done in a steel tank. The tank has a graphite lining on the inner side. This lining does the work of a cathode. A set of graphite rods dipped in the molten electrolyte works as anode. Cryolite (Na3AlF6) and fluorspar (CaF2) are added in the mixture to lower its melting point upto 10000C.
Chapter: [0.08] Metallurgy
Read the following paragraph and answer the questions.
If heat is exchanged between a hot and cold object, the temperature of the cold object goes on increasing due to gain of energy and the temperature of the hot object goes on decreasing due to loss of energy.
The change in temperature continues till the temperatures of both the objects attain the same value. In this process, the cold object gains heat energy and the hot object loses heat energy. If the system of both the objects is isolated from the environment by keeping it inside a heat resistant box (meaning that the energy exchange takes place between the two objects only), then no energy can flow from inside the box or come into the box.
i. Heat is transferred from where to where?
ii. Which principle do we learn about from this process?
iii. How will you state the principle briefly?
iv. Which property of the substance is measured using this principle?
Chapter: [0.05] Heat
Observe the following figure and answer the questions.
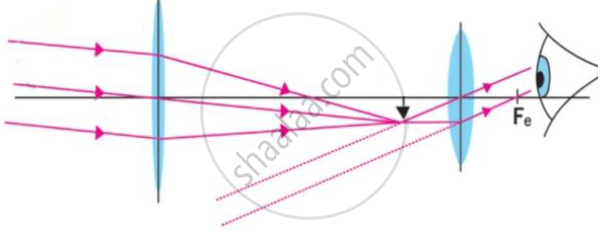
a) Which optical instrument shows arrangement of lenses as shown in the figure?
b) Write in brief the working of this optical instrument.
c) How can we get different magnifications in this optical instrument?
d) Draw the figure again and labelled it properly
Chapter: [0.07] Lenses [0.16] Wonders of Light 1
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
Maharashtra State Board previous year question papers 10th Standard Board Exam Science and Technology 1 with solutions 2018 - 2019
Previous year Question paper for Maharashtra State Board 10th Standard Board Exam -2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science and Technology 1, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of Maharashtra State Board 10th Standard Board Exam.
How Maharashtra State Board 10th Standard Board Exam Question Paper solutions Help Students ?
• Question paper solutions for Science and Technology 1 will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
