Advertisements
Advertisements
Question
Write a molecular formula for rust.
Solution
The chemical formula of rust is Fe2O3•xH2O.
APPEARS IN
RELATED QUESTIONS
Give two examples of alloys with their chemical composition.
Why do we apply paint on iron articles?
Answer the following question.
What are alloys?
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
In one method of rust prevention, the iron is not coated with anything. Which is this method?
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
Name five methods of preventing rusting of iron.
What are the special properties of stainless steel?
Name a common metal which is highly resistant to corrosion.
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Four metals P, Q, R and S are all obtained by the reduction of their oxides with carbon. Metal P is used to form a thin layer over the sheets of metal S to prevent its corrosion. Metal Q is used for electroplating tiffin boxes made of metal S whereas metal R is used in making car batteries. Metals Q and R form an alloy called solder. What are metals P, Q, R and S? How have you arrived at this conclusion?
State under what conditions corrosion is faster ?
Corrosion can be an advantage in some case.Explain ?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
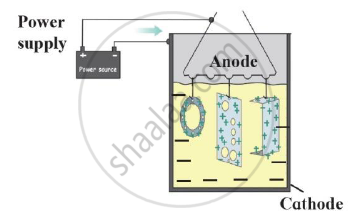
Identify the process shown in the diagram and explain it in short
Write three methods of preventing rusting of iron.
What is "rusting"? Describe with a labelled diagram an activity to investigate the conditions under which iron rusts.
Give reason.
A wooden article should be polished.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
Corrosion of silver causes a black layer of _______.
To prevent corrosion of iron and steel _______ method is used.
Stainless steel is an alloy of _______.
Find the odd one out and give its explanation.
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Complete flow chart given below.

Give preventive methods by giving examples of corrosion?
Amalgam is an alloy of ____________.
The diagram shows the reaction between metal and dil. acid.
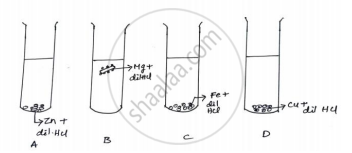
What is the reason for different behaviour of Mg in test tube B?
Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is:
A man painted his main gate made up of iron, to
- prevent it from rusting.
- protect it from the sun.
- make it look beautiful.
- make it dust-free.
Which of the above statement(s) is/are correct?
In ______ process a layer of molten tin is deposited on metals.
