Advertisements
Advertisements
Question
Stainless steel is an alloy of _______.
Options
copper
tin
zinc
iron
Solution
Stainless steel is an alloy of iron.
APPEARS IN
RELATED QUESTIONS
Give two examples of alloys with their chemical composition.
Why do we apply paint on iron articles?
Which of the following methods is suitable for preventing an iron frying pan from rusting?
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
Name the metal which is used for galvanising iron.
In one method of rust prevention, the iron is not coated with anything. Which is this method?
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
Give the constituents and one use of brass.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
What are the constituents of stainless steel?
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
If copper is kept exposed to damp air for a considerable time, it gets a green coating on its surface. This is due to the formation of:
(a) hydrated copper sulphate
(b) copper oxide
(c) basic copper carbonate
(d) copper nitrate
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Bronze is an alloy of ______.
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Answer the following question.
a) What is meant by corrosion?
b) Write names of any two methods of prevention of corrosion.
c) In which method, metal like copper, aluminium are coated with a thin layer of their oxides by means of electrolysis.
d) Explain this method with diagram.
Write three methods of preventing rusting of iron.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
Write a short note on Alloying.
Observe the following picture and answer the following questions:

- What is rust?
- Write the chemical formula of rust.
- Write the reaction of oxidation of iron at the anode.
- Write the reaction of oxidation of iron at the cathode.
- What is corrosion?
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
Explain concept with example/explain with the help of a balanced equation.
Corrosion
Observe the following diagram and give answers.
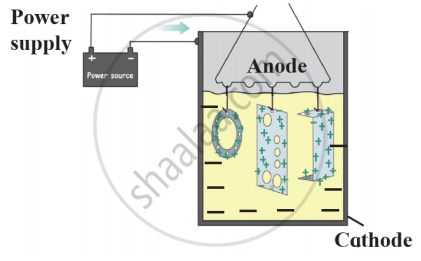
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Give preventive methods by giving examples of corrosion?
What is rust?
Copper objects lose their shine and form green coating of ____________.
The diagram shows the reaction between metal and dil. acid.
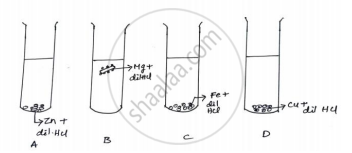
What is the reason for different behaviour of Mg in test tube B?
Identify the correct statement from the following:
Galvanisation is a process used to prevent the rusting of which the following?
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Explain the chemical reactions in rusting of iron.
| A process of forming a thick oxide of aluminium when aluminium is exposed to air. This coat makes it resistant to corrosion. Resistance can be improved by making a layer of oxide thinker. In this technique, the aluminium article is the anode, and the electrolyte is sulphuric acid. The anode reaction results in the formation of a black-coloured film of aluminium oxide on the anode. By putting appropriate dyes in the electrolytic solution, both coloured surface with the decorative finish is achieved. Kitchen articles like anodised such as pressure cookers, pans and frames of sliding windows are applications of this technique. |
- Name the anode and electrolyte used in this technique.
- How can we make aluminium articles made resistant to corrosion?
- Name the technique used to coat the aluminium articles.
Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
