Advertisements
Advertisements
Question
Give the constituents and one use of brass.
Solution
Brass is an alloy of copper and zinc. Composition of brass is copper (80 percent) and zinc (20 percent). Brass is used for making vessels and scientific instruments (e.g.microscope).
APPEARS IN
RELATED QUESTIONS
Explain the terms Corrosion
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
State two conditions for the rusting of iron.
What is the corrosion of iron known as?
Corrosion can be an advantage in some case.Explain ?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
Give reason.
An iron article should be given a coat of paint
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
Write a short note on Alloying.
Observe the following picture and answer the following questions:

- What is rust?
- Write the chemical formula of rust.
- Write the reaction of oxidation of iron at the anode.
- Write the reaction of oxidation of iron at the cathode.
- What is corrosion?
Rusting of iron : Fe2O3 : : corrosion of copper : ______
Find the odd one out and give its explanation.
Write the name.
Method used to prevent corrosion of copper.
Observe the following diagram and give answers.
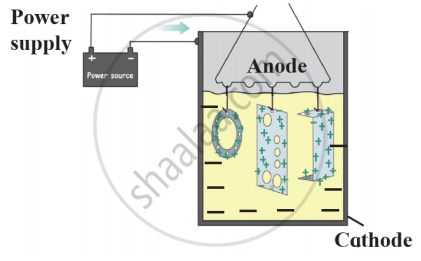
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Give the equation for the formation of rust.
State two conditions necessary for rusting of iron.
The diagram shows the reaction between metal and dil. acid.
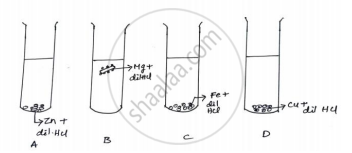
What is the reason for different behaviour of Mg in test tube B?
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
