Advertisements
Advertisements
Question
Rusting of iron : Fe2O3 : : corrosion of copper : ______
Solution
Rusting of iron : Fe2O3 : : corrosion of copper : CuCO3
APPEARS IN
RELATED QUESTIONS
Write two methods of preventing the rusting of iron.
Two methods by which rusting of iron can be prevented are ______ and ______.
Explain how painting of an iron gate prevents it from rusting.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What is anodising? Give its applications.
Name the metal which is used for galvanising iron.
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
What is corrosion?
What is the corrosion of iron known as?
Give the constituents and one use of brass.
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
No chemical reaction takes place when granules of a rusty-brown solid A are mixed with the powder of another solid B. However, when the mixture is heated, a reaction takes place between its components. One of the products C is a metal and settles down in the molten state while the other product D floats over it. It was observed that the reaction is highly exothermic.
(a) What could the solids A and B be?
(b) What are the products C and D most likely to be?
(c) Write the chemical equation for the reaction between A and B leading to the formation of C and D. Mention the physical states of all the reactants and products in this equation and indicate the heat change which takes place.
(d) What is the special name of such a reaction? State one use of such a reaction.
(e) Name any two types of chemical reactions under which the above reaction can be classified.
Four metals P, Q, R and S are all obtained by the reduction of their oxides with carbon. Metal P is used to form a thin layer over the sheets of metal S to prevent its corrosion. Metal Q is used for electroplating tiffin boxes made of metal S whereas metal R is used in making car batteries. Metals Q and R form an alloy called solder. What are metals P, Q, R and S? How have you arrived at this conclusion?
Mention two uses of the following metals and non-metals
Iron
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
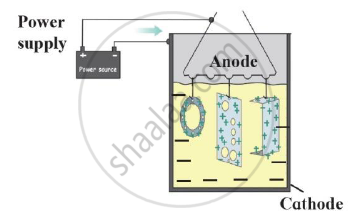
Identify the process shown in the diagram and explain it in short
Find the odd man out:
What is done to prevent corrosion of metals?
Write three methods of preventing rusting of iron.
What is "rusting"? Describe with a labelled diagram an activity to investigate the conditions under which iron rusts.
Give reason.
Copper and brass utensils should be tinned.
Give a reason why rust turns moist red litmus blue.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
Find the odd one out and give its explanation.
Match the columns.
| Group A | Group B |
| 1) Electroplating | a) Pressure cooker |
| 2) Anodising | b) Silver plated spoons |
| c) Coating of tin on copper | |
| d) Coating of Zinc on iron |
Write scientific reason.
Anodization method is useful for prevention of the corrosion of the aluminium.
Observe the following diagram and write answers.

- Name the method.
- Explain the method.
- Give two examples of this method.
Identify the correct statement from the following:
Galvanisation is a process used to prevent the rusting of which the following?
Explain the following:
Lime water turns milky on passing carbon dioxide gas into it.
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Explain the chemical reactions in rusting of iron.
