Advertisements
Advertisements
Question
What is corrosion?
Solution 1
Corrosion is the damage caused to the metal by the chemical reaction of air, water and acids with the surface of the metal. Generally reactive metals corrode easily and non-reactive metals have good resistance to corrosion.
Solution 2
Metals get attacked by substances around it such as moisture, acids, etc. Metal is said to ‘corrode’ due to this attack and the process is called corrosion.
APPEARS IN
RELATED QUESTIONS
What is an alloy?
Tinning : Tin : : Galvanizing : _________
Explain the terms Corrosion
Which metals do not corrode easily?
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What is anodising? Give its applications.
What special name is given to their corrosion of iron?
In one method of rust prevention, the iron is not coated with anything. Which is this method?
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Why is an iron grill painted frequently?
Explain why, through aluminium is more reactive than iron, yet there is less corrosion of aluminium when both are exposed to air.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
Name five methods of preventing rusting of iron.
What are the constituents of stainless steel?
What are the special properties of stainless steel?
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
Four metals P, Q, R and S are all obtained by the reduction of their oxides with carbon. Metal P is used to form a thin layer over the sheets of metal S to prevent its corrosion. Metal Q is used for electroplating tiffin boxes made of metal S whereas metal R is used in making car batteries. Metals Q and R form an alloy called solder. What are metals P, Q, R and S? How have you arrived at this conclusion?
Name the metal which is a constituent of blood pigment?
Compare roasting and calcination.
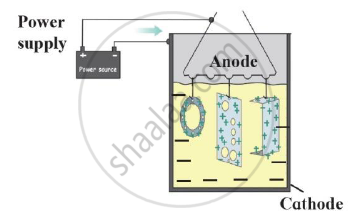
Observe the following picture a write down the chemical reaction with the explanation.

Identify the process shown in the diagram and explain it in short
Choose the correct alternative and rewrite the following:
Iron is _____________________.
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
_______ is an alloy made from iron, carbon and chromium.
Corrosion of silver causes a black layer of _______.
To prevent corrosion of iron and steel _______ method is used.
Pressure cooker : Anodizing : : Silver plated spoons : _______
Rusting of iron : Fe2O3 : : corrosion of copper : ______
Bronze : _______ : : Stainless steel : Fe + Cr + C
Find the odd one out and give its explanation.
Write the name.
An alloy of copper and tin-
Write scientific reason.
Anodization method is useful for prevention of the corrosion of the aluminium.
Write scientific reason.
Coins are made from metals and alloys.
Write a molecular formula for rust.
Observe the following diagram and give answers.
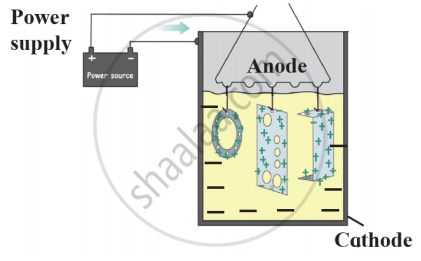
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
The process of coating the surface of the metal with a thin layer of zinc is called ______
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
Gold plated ornaments is the example of ______.
Give scientific reasons.
Silver amalgam is used for filling dental cavities.
