Advertisements
Advertisements
Question
Pressure cooker : Anodizing : : Silver plated spoons : _______
Solution
Pressure cooker : Anodizing : : Silver plated spoons : Electroplating
APPEARS IN
RELATED QUESTIONS
Why do we apply paint on iron articles?
Explain the terms Corrosion
Which metals do not corrode easily?
Explain how painting of an iron gate prevents it from rusting.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What type of chemical reaction is involved in the corrosion of iron?
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
Fill in the following blank with suitable word:
The corrosion of iron is called ................
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
What is the corrosion of iron known as?
Give the constituents and one use of brass.
Name five methods of preventing rusting of iron.
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Name the metal which is a constituent of plant pigment?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Observe the following picture a write down the chemical reaction with the explanation.

Identify the process shown in the diagram and explain it in short
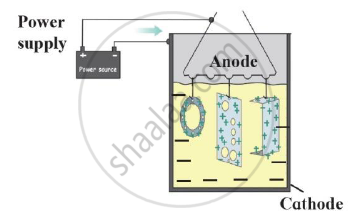
What are the adverse effects of corrosion?
Choose the correct alternative and rewrite the following:
Iron is _____________________.
Give reason.
A wooden article should be polished.
Give reason.
Copper and brass utensils should be tinned.
Give a reason why rust turns moist red litmus blue.
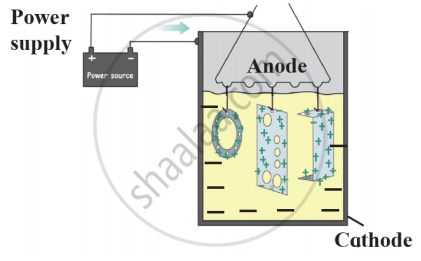
Observe the following picture and answer the following questions:

- What is rust?
- Write the chemical formula of rust.
- Write the reaction of oxidation of iron at the anode.
- Write the reaction of oxidation of iron at the cathode.
- What is corrosion?
When one of the metals in an alloy is mercury the alloy is called _______.
Find the odd one out and give its explanation.
Write the name.
Method used to prevent corrosion of copper.
Complete flow chart given below.

Observe the following diagram and give answers.
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Observe the following figure and write the answer of the question.

- Which process is shown in the figure?
- Explain the chemical reaction shown in the figure.
- Write the reactions on anode and cathode.
Give preventive methods by giving examples of corrosion?
Amalgam is an alloy of ____________.
Which among the following alloys contain mercury as one of its constituents?
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
Describe two changes that are harmful. Explain why you consider them harmful. How can you prevent them?
