Advertisements
Advertisements
Question
Give reason.
An iron article should be given a coat of paint
Solution
Iron articles often undergo rusting, resulting in the formation of a reddish-brown layer on their surface. Rusting is caused by the exposure of iron to the air and water in the surroundings. An iron article that is coated with paint prevents the interaction of iron with the surrounding air and water and thus protects it from rusting.
RELATED QUESTIONS
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
Name any two metals which do not corrode easily.
Explain why, the galvanised iron article is protected against rusting even if the zinc layer is broken.
Identify the process shown in the diagram and explain it in short
_______ is an alloy made from iron, carbon and chromium.
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
Explain concept with example/explain with the help of a balanced equation.
Corrosion
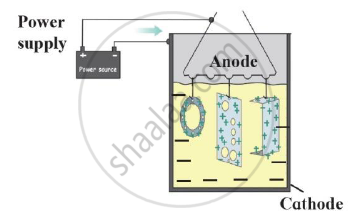
Observe the following diagram and give answers.
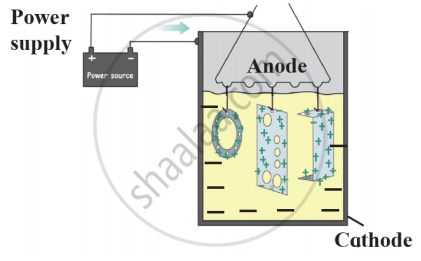
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Copper objects lose their shine and form green coating of ____________.
