Advertisements
Advertisements
Questions
Answer the following question.
What are alloys?
Answer the following question.
What are alloys? Give two examples
Solution 1
An alloy is the homogeneous mixture of two or more metals or metals and non-metals. For example, brass is an alloy of copper and zinc.
For example:
- Stainless steel is an alloy of Nickel and Chromium.
- Amalgam is an alloy of Mercury.
- Brass is an alloy of Copper and Zinc.
- Bronze is an alloy of Copper and Tin.
- Solder is an alloy of Lead and Tin.
Solution 2
An alloy is a homogenous mixture of two or more metals or a metal and a non–metal in definite proportion.
Example:
- Brass – copper, and zinc.
- Stainless steel – iron, nickel, and chromium
RELATED QUESTIONS
What is an alloy?
Give two examples of alloys with their chemical composition.
Tinning : Tin : : Galvanizing : _________
Explain the terms Corrosion
Two methods by which rusting of iron can be prevented are ______ and ______.
Explain how painting of an iron gate prevents it from rusting.
Explain why Iron sheets are coated with zinc during galvanization.
What is anodising? Give its applications.
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Explain why, through aluminium is more reactive than iron, yet there is less corrosion of aluminium when both are exposed to air.
What is corrosion?
What is the corrosion of iron known as?
Name five methods of preventing rusting of iron.
Name an alloy of copper. State its chemical composition and any one use.
Name a common metal which is highly resistant to corrosion.
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Brass is an alloy of:
(a) Cu and Sn
(b) Cu and Pb
(c) Pb and Sn
(d) Zn and Cu
No chemical reaction takes place when granules of a rusty-brown solid A are mixed with the powder of another solid B. However, when the mixture is heated, a reaction takes place between its components. One of the products C is a metal and settles down in the molten state while the other product D floats over it. It was observed that the reaction is highly exothermic.
(a) What could the solids A and B be?
(b) What are the products C and D most likely to be?
(c) Write the chemical equation for the reaction between A and B leading to the formation of C and D. Mention the physical states of all the reactants and products in this equation and indicate the heat change which takes place.
(d) What is the special name of such a reaction? State one use of such a reaction.
(e) Name any two types of chemical reactions under which the above reaction can be classified.
Mention two uses of the following metals and non-metals
Iron
Name the metal which is a constituent of plant pigment?
State under what conditions corrosion is faster ?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
Answer the following question.
a) What is meant by corrosion?
b) Write names of any two methods of prevention of corrosion.
c) In which method, metal like copper, aluminium are coated with a thin layer of their oxides by means of electrolysis.
d) Explain this method with diagram.
Find the odd man out:
Give reason.
An iron article should be given a coat of paint
Give reason.
A wooden article should be polished.
Give reason.
Copper and brass utensils should be tinned.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
Give a reason why rust turns moist red litmus blue.
_______ is an alloy made from iron, carbon and chromium.
To prevent corrosion of iron and steel _______ method is used.
_______ forms a green colour in the water.
Stainless steel is an alloy of _______.
Pressure cooker : Anodizing : : Silver plated spoons : _______
Bronze : _______ : : Stainless steel : Fe + Cr + C
Find the odd one out and give its explanation.
Find the odd one out and give its explanation.
Write scientific reason.
Coins are made from metals and alloys.
Draw a neat labelled diagram.
Electroplating
Draw a neat labelled diagram.
Anodizing
Observe the following diagram and give answers.
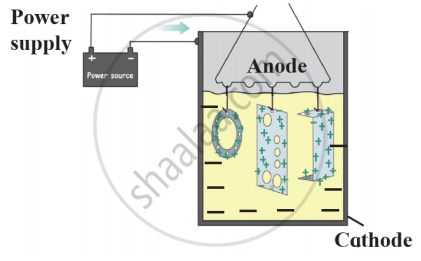
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
What is rust?
Copper objects lose their shine and form green coating of ____________.
Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is:
Identify the correct statement from the following:
A man painted his main gate made up of iron, to
- prevent it from rusting.
- protect it from the sun.
- make it look beautiful.
- make it dust-free.
Which of the above statement(s) is/are correct?
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
| A process of forming a thick oxide of aluminium when aluminium is exposed to air. This coat makes it resistant to corrosion. Resistance can be improved by making a layer of oxide thinker. In this technique, the aluminium article is the anode, and the electrolyte is sulphuric acid. The anode reaction results in the formation of a black-coloured film of aluminium oxide on the anode. By putting appropriate dyes in the electrolytic solution, both coloured surface with the decorative finish is achieved. Kitchen articles like anodised such as pressure cookers, pans and frames of sliding windows are applications of this technique. |
- Name the anode and electrolyte used in this technique.
- How can we make aluminium articles made resistant to corrosion?
- Name the technique used to coat the aluminium articles.
