English Medium
Academic Year: 2018-2019
Date: March 2019
Advertisements
- Question numbers 1 and 2 in Section A are one mark questions.
- Question numbers 3 to 5 in Section B are two marks questions.
- Question numbers 6 to 15 in Section C are three-marks questions.
- Question numbers 16 to 21 in Section D are five-marks questions.
- Question numbers 22 to 27 in Section E are based on practical skills. Each question is a two marks question.
Name and define the SI unit of current.
Chapter: [0.12] Magnetic Effects of Electric Current
Answer the following question.
Write the name of the main constituent of Biogas. Also, state its percentage.
Chapter: [0.14] Sources of Energy
Answer the following question.
Write the name, symbol, and electronic configuration of an element X whose atomic number is 11.
Chapter: [0.05] Periodic Classification of Elements
Can the following groups of elements be classified as Dobereiner's triad:
Na, Si, Cl
Atomic mass of Na - 23, Si - 28, Cl - 35.
Justify your answer.
Chapter: [0.05] Periodic Classification of Elements
Can the following groups of elements be classified as Dobereiner's triad:
Be, Mg, Ca
Atomic mass of Be - 9, Mg - 24, Ca - 40.
Justify your answer.
Chapter: [0.05] Periodic Classification of Elements
Answer the following question.
How is O2 and CO2 transported in human beings?
Chapter: [0.05] Life Processes [0.05] Life Processes
Answer the following question.
Write the structure of eye lens and state the role of ciliary muscles in the human eye.
Chapter: [0.1] The Human Eye and the Colourful World
Answer the following question.
Identify the acid and base which form sodium hydrogen carbonate. Write the chemical equation in support of your answer. State whether this compound is acidic, basic, or neutral. Also, write its pH value.
Chapter: [0.02] Acids, Bases and Salts
Based on the group valency of element write the molecular formula of the following compound giving justification:
Oxide of first group elements.
Chapter: [0.05] Periodic Classification of Elements
Based on the group valency of element write the molecular formula of the following compound giving justification:
Halide of the elements of group thirteen
Chapter: [0.05] Periodic Classification of Elements
Based on the group valency of element write the molecular formula of the following compound giving justification:
Compound formed when an element A of group 2 combines with an element B of group seventeen.
Chapter: [0.05] Periodic Classification of Elements
Answer the following question.
2 g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Chapter: [0.01] Chemical Reactions and Equations
Identify the type of reaction taking place in the following case and write the balanced chemical equation for the reaction.
Zinc reacts with silver nitrate to produce zinc nitrate and silver.
Chapter: [0.01] Chemical Reactions and Equations
Identify the type of reaction taking place in the following case and write the balanced chemical equation for the reaction.
Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
Chapter: [0.01] Chemical Reactions and Equations
Define the following Term:
Transpiration
Chapter: [0.05] Life Processes
Design an experiment to demonstrate the transpiration process.
Chapter: [0.05] Life Processes
Answer the following question.
What is the feedback mechanism of harmonic regulation? Take the example of insulin to explain this phenomenon.
Chapter: [0.06] Control and Co-ordination
Answer the following question.
What are plant hormones? Name the plant hormones responsible for the following:
- Growth of stem
- Promotion of cell division
- Inhibition of growth
- Elongation of cells
Chapter: [0.06] Control and Co-ordination
Advertisements
Answer the following question.
Name the plant Mendel used for his experiment. What type of progeny was obtained by Mendel in F1 and F2 generations when he crossed the tall and short plants? Write the ratio he obtained in F2 generation plants.
Chapter: [0.08] Heredity
List two differences between acquired traits and inherited traits by giving an example of each.
Chapter: [0.08] Heredity
Answer the following question.
Why should there be an equitable distribution of resources? List three forces that would be working against an equitable distribution of our resources.
Chapter: [0.16] Sustainable Management of Natural Resources
Answer the following question.
How can we help in reducing the problem of waste disposal? Suggest any three methods.
Chapter: [0.13] Our Environment
Answer the following question.
Define an ecosystem. Draw a block diagram to show the flow of energy in an ecosystem.
Chapter: [0.13] Our Environment
What is a rainbow? Draw a labelled diagram to show the formation of a rainbow.
Chapter: [0.1] The Human Eye and the Colourful World
Answer the following question.
Write the chemical formula and name of the compound which is the active ingredient of all alcoholic drinks. List its two uses.
Write the chemical equation and name of the product formed when this compound reacts with -
(i) Sodium metal
(ii) hot concentrated sulphuric acid
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
What is methane?
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
Draw methane electron dot structure.
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
Name the type of bonds formed in the methane compound.
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
Why are methane compounds have poor conductors of electricity?
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
Why are methane compounds have low melting and boiling points?
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
What happens when methane compound burns in oxygen?
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following reaction:
Calcium metal reacts with water.
Chapter: [0.03] Metals and Non Metals
Write the chemical equation for the following reaction:
Cinnabar is heated in the presence of air.
Chapter: [0.03] Metals and Non Metals
Write the chemical equation for the following reaction:
Manganese dioxide is heated with aluminum powder.
Chapter: [0.03] Metals and Non Metals
Answer the following question.
What are alloys?
Chapter: [0.01] Chemical Reactions and Equations [0.03] Metals and Non Metals
Answer the following question.
List two properties of alloys.
Chapter: [0.01] Chemical Reactions and Equations [0.03] Metals and Non Metals
Answer the following question:
An object is placed at a distance of 30 cm from a concave lens of focal length 30 cm.
(i) Use the lens formula to determine the distance of the image from the lens.
(ii) List four characteristics of the image (nature, position, size, erect/inverted) in this case.
(iii) Draw a labelled diagram to justify your answer of the part (ii)
Chapter: [0.09] Light - Reflection and Refraction
Answer the following question.
With the help of a suitable circuit diagram prove that the reciprocal of the equivalent resistance of a group of resistances joined in parallel is equal to the sum of the reciprocals of the individual resistances.
Chapter: [0.11] Electricity
Advertisements
Solve the following question:
In an electric circuit, two resistors of 12 0 each are joined in parallel to a 6 V battery. Find the current drawn from the battery.
Chapter: [0.11] Electricity
Answer the following question.
An electric lamp of resistance 20 Ω and a conductor of resistance 4 0 are connected to a 6 V battery as shown in the circuit. Calculate: 
(a) the total resistance of the circuit,
(b) the current through the circuit,
(c) the potential difference across the (i) electric lamp and (ii) conductor, and
(d) power of the lamp.
Chapter: [0.11] Electricity
Answer the following question.
Draw the pattern of magnetic field lines of
(i) a current-carrying solenoid and
(ii) a bar magnet.
List two distinguishing features between the two fields.
Chapter: [0.12] Magnetic Effects of Electric Current
Answer the following question.
How does suitable pollination lead to fertilization?
Chapter: [0.07] How do Organisms Reproduce?
Identify the given diagram.
Name the parts 1 to 5. 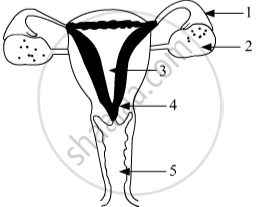
Chapter: [0.07] How do Organisms Reproduce?
Answer the following question.
What is contraception? List three advantages of adopting contraceptive measures.
Chapter: [0.07] How do Organisms Reproduce?
Answer the following question.
In the experimental set up to show that "CO2 is given out during respiration", name the substance taken in the small test tube kept in the conical flask. State its function and the consequence of its use.
Chapter: [0.05] Life Processes [0.05] Life Processes
A student is observing the temporary mount of a leaf peel under a microscope. Draw a labelled diagram of the structure of stomata as seen under the microscope
Chapter: [0.07] How do Organisms Reproduce?
Draw a labelled diagram in proper sequence to show budding in hydra.
Chapter: [0.07] How do Organisms Reproduce?
Answer the following question.
List four precautions which a student should observe while determining the focal length of a given convex lens by obtaining an image of a distant object on a screen.
Chapter: [0.09] Light - Reflection and Refraction
Answer the following question.
While studying the dependence of potential difference ( V) across a resistor on the current (I) passing through it, in order to determine the resistance of the resistor, a student took 5 readings for different values of current and plotted a graph between V and t. He got a straight line graph passing through the origin. What does the straight-line signify? Write the method of determining the resistance of the resister using this graph.
Chapter: [0.11] Electricity
What would you suggest to a student if while performing an experiment he finds that the pointer/needle of the ammeter and voltmeter do not coincide with the zero marks on the scales when the circuit is open? No extra ammeter/voltmeter is available in the laboratory.
Chapter: [0.11] Electricity
Answer the following question.
In three test tubes A, B, and C, three different liquids namely, distilled water, underground water and distilled water in which a pinch of calcium sulphate is dissolved, respectively are taken. Equal amount of soap Answer is added to each test tube and the contents are shaken. In which test tube will the length of the foam (lather) be longest? Justify your answer.
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
Blue litmus solution is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube a colour change will be observed? State the colour change and give its reason.
Chapter: [0.02] Acids, Bases and Salts
Answer the following question.
What is observed when 2 mL of dilute hydrochloric acid is added to 1 g of sodium carbonate taken in a clean and dry test tube? Write a chemical equation for the reaction involved.
Chapter: [0.01] Chemical Reactions and Equations
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2018 - 2019
Previous year Question paper for CBSE Class 10 Science-2019 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
