Advertisements
Advertisements
Question
Write the chemical equation for the following reaction:
Cinnabar is heated in the presence of air.
Solution
\[\ce{\underset{\text{Cinnabar}}{HgS}+\underset{\text{Oxygen from air}}{3O2}->\underset{\text{Mercury(ll) oxide}}{2HgO}+\underset{\text{Sulphur dioxide}}{2SO2}}\]
\[\ce{\underset{\text{Mercury(ii) oxide}}{2HgO}->\underset{\text{Mercury metal}}{2Hg}+\underset{\text{Oxygen gas}}{O2}}\]
In the above reaction when cinnabar is heated in the presence of oxygen, it is reduced to produce pure mercury metal.
APPEARS IN
RELATED QUESTIONS
Write equations for the reactions of calcium and potassium with water.
Which gas is produced when dilute hydrochloric acid is added to a reactive metal?
Complete and balance the following equation:
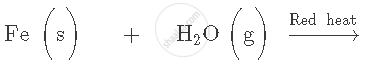
What is the nature of the oxide Na2O? What happens when it is dissolved in water? Write the chemical equation of the reaction involved.
You are given a solution of AgNO3. Which of the following do you think cannot displace Ag from AgNO3solution?
(a) Magnesium
(b) Zinc
(c) Gold
(d) Copper
CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s)
FeSO4(aq) + Zn(s) → ZnSO4(aq) + Fe(s)
On the basis of the above reactions, indicate which is most reactive and which is least reactive metal out of zinc, copper and iron.
What is the characteristic of the electronic configuration of noble gases?
Write the name and formula of a molecule made up of three atoms of oxygen.
An alkali metal A gives a compound B (molecular mass = 40) on reacting with water. The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A, B and C and give the reaction involved.
The substance that will be flattened on beating with a hammer is
