Advertisements
Advertisements
Question
Complete and balance the following equation:
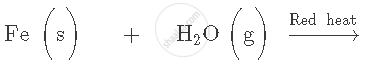
Solution
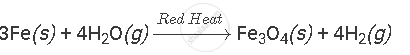
APPEARS IN
RELATED QUESTIONS
Explain the following reaction with the help of a balanced chemical equation:
Magnesium reacts with hot water.
What will happen if a strip of copper is kept immersed in a solution of silver nitrate (AgNO3)?
How would you show that silver is chemically less reactive than copper?
How do non-metals react with hydrogen? Explain with an example.
What happens when calcium reacts with water? Write the chemical equation of the reaction of calcium with eater.
You are given a solution of AgNO3. Which of the following do you think cannot displace Ag from AgNO3solution?
(a) Magnesium
(b) Zinc
(c) Gold
(d) Copper
An element X forms two oxides XO and XO2. The oxide XO has no action on litmus solution but oxide XO2 turns litmus solution red.
(a) What is the nature of oxide XO?
(b) What is the nature of oxide XO2?
(c) Would you call element X a metal or a non-metal? Give reason for your choice.
(d) Can you give an example of element like X?
In nature, metal A is found in a free state while metal B is found in the form of its compounds. Which of these two will be nearer to the top of the activity series of metals?
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
- Good thermal conductivity
- Good electrical conductivity
- Ductility
- High melting point
Metals are generally hard. Which of the following metals is an exception and can be cut with a knife?
