Advertisements
Advertisements
Question
Answer the following question.
List two properties of alloys.
Solution
Properties of alloys:
- Alloys are stronger than the metals from which they are made.
- Alloys are more resistant to corrosion.
APPEARS IN
RELATED QUESTIONS
Give reasons:
Platinum, gold and silver are used to make jewellery.
What is anodising? Give its applications.
Fill in the following blank with suitable word:
The corrosion of iron is called ................
What is the corrosion of iron known as?
A metal X which is resistant to corrosion is produced by the electrolysis of its molten oxide whereas another metal Y which is also resistant to corrosion is produced by the reduction of its oxide with carbon. Metal X can be used in powder form in thermite welding whereas metal Y is used in making cathodes of ordinary dry cells.
(a) Name the metals X and Y.
(b) Which of the two metals is more reactive : X or Y?
(c) Name one ore or metal X. Also write its chemical formula.
(d) Name one ore or metal Y. Also write its chemical formula.
(e) Name one alloy of metal X and one alloy of metal Y.
Bronze is an alloy of ______.
Name the metal which is a constituent of blood pigment?
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
What are the adverse effects of corrosion?
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
Write a short note on Alloying.
_______ is an alloy made from iron, carbon and chromium.
Write the name.
Method used to prevent corrosion of copper.
Observe the following diagram and give answers.
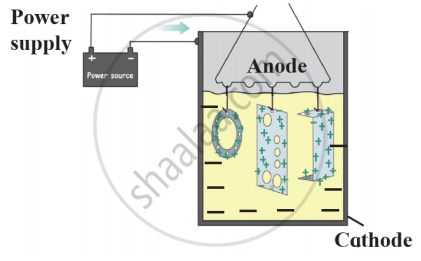
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Give preventive methods by giving examples of corrosion?
State two conditions necessary for rusting of iron.
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
The iron pillar near the Qutub Minar in Delhi is famous for the following facts. Which of these facts is responsible for its long stability?
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Gold plated ornaments is the example of ______.
