Advertisements
Advertisements
Question
Based on the group valency of element write the molecular formula of the following compound giving justification:
Oxide of first group elements.
Solution
Oxides of the first group elements have the common formula of M20.
Example- Na20, K20. This is because, the first group elements have a common valency of 1, and the valency of Oxygen is 2 so, to satisfy the combining capacity of Oxygen two 1st group metals are required. 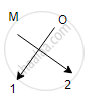
APPEARS IN
RELATED QUESTIONS
How do the valency and the atomic size of the elements vary while going from left to right along a period in the modern periodic table?
Write any one difference in the electronic configurations of group-1 and group-2 elements ?
The metal of Group 2 from top to bottom arc Be, Mg, Ca, Sr, and Ba.
1) Which one of these elements will form ions most readily and why?
2) State the common feature in the electronic configuration of all these elements.
The atomic radii of three elements X, Y and Z of a period of the periodic table are 186 pm; 104 pm and 143 pm respectively. Giving a reason, arrange these elements in the increasing order of atomic numbers in the period.
How can the valency of an element be determined if its electronic configuration is known?
What will be the valency of an element of atomic number 9 (nine)?
Choose the most appropriate answer from the following list of oxides which fit the description.
A basic oxide.
The elements of one short period of the periodic table are given below in order from left to right:
| Li | Be | B | C | O | F | Ne |
Place the three elements fluorine, beryllium and nitrogen in the order of increasing electronegativity.
Name or state following with reference to the elements of the first three periods of the periodic table.
The number of electron shells in elements of period 1, period 2, and period 3.
Name or state following with reference to the element of the first three periods of the periodic table.
A covalent compound formed between an element in period 1 and a halogen.
