Advertisements
Advertisements
Question
Describe the extraction of zinc metal from its sulphide ore (zinc blende). Write equations of the reactions involved.
Solution
Zinc blende (ZnS) is the sulphide ore of zinc. The extraction of zinc from zinc blende involves two steps. First, the concentrated zinc blende is converted to its oxide (by roasting). After this, the oxide is reduced to zinc metal. The reactions for the two processes are:
1. Roasting: Zinc blende is subjected to roasting, i.e. it is heated in the presence of excess air. This converts zinc sulphide ore to zinc oxide.
The reaction involved is:

Reduction: Zinc oxide is then reduced by coke (carbon). When zinc oxide is heated with coke, carbon acts as a reducing agent and reduces zinc oxide to zinc metal.
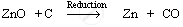
APPEARS IN
RELATED QUESTIONS
Write the composition of the alloy called bronze.
Which metal is extracted from calamine ore?
How is copper extracted from its sulphide ore (copper glance), Cu2S? Explain with equations of the reactions involved.
The metal which can be extracted from the bauxite ore is:
(a) Na
(b) Mn
(c) Al
(d) Hg
The metal which is always present in an amalgam is:
(a) iron
(b) aluminium
(c) mercury
(d) magnesium
Define the following term.
Mineral
Name the following:
Name two metals always find in combined state.
Usually ______ ores are subjected to ______ which is done in the absence of air.
In the electrolytic reduction of alumina, the lining of graphite acts as an anode.
Care must be taken while diluting concentrated nitric acid with water. Why?
