Advertisements
Advertisements
प्रश्न
Describe the extraction of zinc metal from its sulphide ore (zinc blende). Write equations of the reactions involved.
उत्तर
Zinc blende (ZnS) is the sulphide ore of zinc. The extraction of zinc from zinc blende involves two steps. First, the concentrated zinc blende is converted to its oxide (by roasting). After this, the oxide is reduced to zinc metal. The reactions for the two processes are:
1. Roasting: Zinc blende is subjected to roasting, i.e. it is heated in the presence of excess air. This converts zinc sulphide ore to zinc oxide.
The reaction involved is:

Reduction: Zinc oxide is then reduced by coke (carbon). When zinc oxide is heated with coke, carbon acts as a reducing agent and reduces zinc oxide to zinc metal.
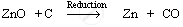
APPEARS IN
संबंधित प्रश्न
In the extraction of aluminium: Name the process of concentration of bauxite.
In the extraction of aluminium: Write the function and formula of cryolite in the extraction of aluminium.
Why are the metals like Na, K, Ca and Mg never found in their free state in nature?
Which one of the methods given in column I is applied for the extraction of each of the metals given in column II:
| Column I | Column II |
| Electrolytic reduction | Aluminium |
| Reduction with Carbon | Zinc |
| Reduction with Aluminium | Sodium |
| Iron | |
| Manganese | |
| Tin |
Give the name of one ore of iron. Which iron compound is present in this ore? Write its chemical formula.
Complete the incomplete statement with missing words:
Metals are ______ while non-metals are ______ conductors of heat.
Bauxite reacts with sodium hydroxide in the Bayer’s process.
During extraction of metals, electolytic refining is used to obtain pure metals.
(a) Which material will be used as anode and cathode for refining of silver metal by this process?
(b) Suggest a suitable electrolyte also.
(c) In this electrolytic cell, where do we get pure silver after passing electric current?
The given reaction shows one of the processes to extract the metals like Iron and Manganese.
\[\ce{MnO2(s) + Al(s) -> Mn(l) + Al2O3(s) + Heat}\]
Give the reason why the above reaction is known as a thermite reaction.
A mineral from which the metal can be extracted economically and conveniently is known as ______.
