Advertisements
Advertisements
प्रश्न
Describe the extraction of zinc metal from its sulphide ore (zinc blende). Write equations of the reactions involved.
उत्तर
Zinc blende (ZnS) is the sulphide ore of zinc. The extraction of zinc from zinc blende involves two steps. First, the concentrated zinc blende is converted to its oxide (by roasting). After this, the oxide is reduced to zinc metal. The reactions for the two processes are:
1. Roasting: Zinc blende is subjected to roasting, i.e. it is heated in the presence of excess air. This converts zinc sulphide ore to zinc oxide.
The reaction involved is:

Reduction: Zinc oxide is then reduced by coke (carbon). When zinc oxide is heated with coke, carbon acts as a reducing agent and reduces zinc oxide to zinc metal.
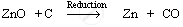
APPEARS IN
संबंधित प्रश्न
Define the Ore.
What chemical process is used for obtaining a metal from its oxide?
The metal which can be extracted from the bauxite ore is:
(a) Na
(b) Mn
(c) Al
(d) Hg
Manganese metal is extracted from manganese dioxide by a reduction process by making use of:
(a) carbon
(b) hydrogen
(c) electrolysis
(d) aluminium
The metal which can be extracted simply by heating the cinnabar ore in air is:
(a) Zn
(b) Cu
(c) Al
(d) Hg
How an ore is concentrated by froth floatation process?
Draw a well-labelled diagram of extraction of Aluminium. Write the anode reaction in electrolytic reduction of Alumina.
The electrolysis of alumina involves the use of fluorspar and cryolite to increase the melting point.
Explain the following reaction with the balanced equation.
Chlorine dissolved in water
In thermite welding a mixture of ____________ is ignited with a burning magnesium ribbon which produces molten iron metal as a large amount of heat is evolved.
