Advertisements
Advertisements
प्रश्न
How an ore is concentrated by froth floatation process?
उत्तर
Concentrated of an ore by froth floatation process : This process depends on preferential wettability of the ore and the gangue particles. Crushed ore is taken in
a large tank containing water and certain oils. The ore particles get wetted by the oil and the gangue particles get wetted by water. The mixture is then agitated with
the help of compressed air. The ore particles that get wetted with the oil form a froth on the top, and can be scooped out. This method is used for the concentration
of sulphide ores.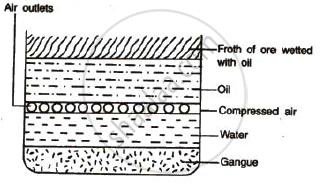
Figure : Froth floatation process
APPEARS IN
संबंधित प्रश्न
The process by which sulphide ore is concentrated.
How is sodium metal extracted? Explain with the help of equation of the reaction involved.
Name three other metals which are extracted in a manner similar to sodium.
An element has its electron configuration as 2,8,8,2. Now answer the following questions.
a) What is the atomic number of this element?
b) What is the group of this element?
c) To which period does this element belong?
State three objectives achieved during the roasting of ores.
Draw a well-labelled diagram of extraction of Aluminium. Write the anode reaction in electrolytic reduction of Alumina.
Write the name.
The molecular formula of main ore of aluminium –
Write the molecular formulae of the following compound.
Sodium aluminate
Explain the concept of Roasting.
Iqbal treated a lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how can he identify the produced gas. Write chemical equations for both the reactions.
