Advertisements
Advertisements
Question
Give one example of an endothermic reaction.
Solution
Example of endothermic reaction: Barium hydroxide on reacting with ammonium chloride produces ammonia, water and barium chloride. It also results in decrease in temperature, as it consumes heat energy.
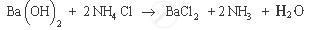
APPEARS IN
RELATED QUESTIONS
What is a balanced chemical equation? Why should a chemical equation be balanced?
Balance the given equation:
H2O2  H2O + O2
H2O + O2
Is Burning of natural gas an endothermic reaction or an exothermic reaction?
Write balanced chemical equation with state symbols for the following reaction:
Barium chloride solution reacts with sodium sulphate solution to give insoluble barium sulphate and a solution of sodium chloride.
Write your observation for the following chemical reaction and name the product formed :
When an aqueous solution of sodium chloride is mixed with an aqueous solution of silver nitrate.
To determine the percentage of water absorbed by raisins, raisins are soaked in water for:
In certain reaction a change of state is observed i.e. solid to liquid, liquid to gas etc. – State the change of state of the products – to give the respective reactant.
\[\ce{C +2S -> CS2}\]
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
What is the indications of the arrow between the reactants and the products and of the arrow pointing downwards at the end.
Balance the following simple equation:
NO + O2 → NO2
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
Na2SO3 + HCl → 2NaCl + H2O + SO2
