Advertisements
Advertisements
Question
How will you indicate a solution made in water in a chemical equation?
Solution
A solution made in water is indicated by writing (aq) after its formula in a chemical equation.
Example: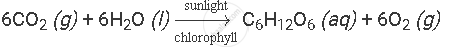
APPEARS IN
RELATED QUESTIONS
Write the balanced chemical equation for the following reaction.
\[\ce{Zinc + Silver nitrate -> Zinc nitrate + Silver}\]
Balance the following equation. Also name the product formed.
` "H"_2"O"_2 → "H" _2 "O" + "O"_2`
What do the following symbol stand for?
2H2
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate mass of MnO2 used.
A student mixes sodium sulphate powder in barium chloride powder. What change would the student observe on mixing the two powders? Justify your answer and explain how he can obtain the desired change.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – copper carbonate.
An element X is trivalent. Write the balanced equation for the combustion of X in oxygen.
Explain the term ‘chemical equation’.
Balance the following simple equation:
K + CO2 → K2O + C
Write the skeleton equation for the following word equation and then balance them.
- Carbon + Oxygen → Carbon dioxide.
- Phosphorus + Chlorine → Phosphorus pentachloride.
- Sulphur + Oxygen → Sulphur dioxide.
- Magnesium + hydrogen → Magnesium + Hydrogen chloride chloride.
