Advertisements
Online Mock Tests
Chapters
2: Acids, Bases and Salts
3: Metals and Non-metals
▶ 4: Carbon And Its Compounds
5: Periodic Classification Of Elements
![Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 4 - Carbon And Its Compounds Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 4 - Carbon And Its Compounds - Shaalaa.com](/images/9352831810-chemistry-science-english-class-10_6:7eeaf5db0f4a49c580d3c8746ff5ab36.jpg)
Advertisements
Solutions for Chapter 4: Carbon And Its Compounds
Below listed, you can find solutions for Chapter 4 of CBSE Lakhmir Singh for Chemistry (Science) [English] Class 10.
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 4 Carbon And Its Compounds Exercise 1 [Pages 220 - 225]
Name the element whose one of the allotropic forms is buckminsterfullerene.
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
State whether the following statement is true or false:
Diamond and graphite are the covalent compounds of carbon element (C)
Name the scientist who disproved the 'vital force theory' for the formation of organic compounds.
Name the element whose allotropic form is graphite.
In addition to some propane and ethane, LPG cylinders contain mainly two isomers of another alkane. Name the two isomers and write their condensed structural formulae.
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many hexagons of carbon atoms are present in one molecule of buckminsterfullerene?
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many pentagons of carbon atoms are present in one molecule of buckminsterfullerene?
Name the black substance of pencil. Will the current flow through the electrical circuit when we use the sharpened ends of the pencil to complete the circuit?
How does graphite act as a lubricant?
Name the hardest natural substance known.
Which of the following molecule is called buckministerfullerene?
C90 C60 C70 C120
Give the name and structural formula of an alkyl group.
Write the electron-dot structures for ethane.
Write the electron-dot structures for ethene.
Write the electron-dot structures for ethyne.
Give the IUPAC name of the following compound:
C2H6
Write the structural formula of propene.
Write the structural formula of propyne.
Write the structural formula of butane.
What do you call the compounds having the same molecular formula but different structural arrangements of atoms?
Write the names of any two isomers represented by the molecular formula C5H12.
Write down (i) structural formula, of any one isomer of hexane (C6H14), other than n-hexane.
Write down electron-dot formula, of any one isomer of hexane (C6H14), other than n-hexane.
Fill in the following blank with suitable word:
The form of carbon which is known as black lead is ...........
Fill in the following blank with suitable word:
The general formula CnH2n for cycloalkanes is the same as that of ...........
Fill in the following blank with suitable word:
The IUPAC name of ethylene is .........
Fill in the following blank with suitable word:
The IUPAC name of acetylene is ............
Fill in the following blank with suitable word:
The form of carbon which is used as a lubricant at high temperature is .........
Fill in the following blank with suitable word:
Compounds of carbon with hydrogen alone are called ..........
Fill in the following blank with suitable word:
CnH2n is the general formula of .......... hydrocarbons.
Fill in the following blank with suitable word:
Hydrocarbons having the general formula CnH2n−2 are called ..........
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
Fill in the following blank with suitable word:
Ethyne has ......... carbon-hydrogen single bonds.
Fill in the following blank with suitable word:
Carbon compounds have usually ... melting points and boiling points because they are ...... in nature.
Fill in the following blank with suitable word:
The property of carbon atoms to form long chains in compounds is called ...........
What is the atomic number of carbon. Write its electronic configuration.
What type of chemical bonds are formed by carbon? Why?
Name the three allotropic forms of carbon.
What is the general name of all the compounds made up of carbon and hydrogen?
Why does carbon form compounds mainly by covalent bonding?
Name two elements which exhibit the property of catenation.
What is meant by catenation?
Write the names and structural formulae of all the possible isomers of hexane.
What is buckminsterfullerene?
How buckminsterfullerene is it related to diamond and graphite?
Why is diamond used for making cutting tools (like glass cutters) but graphite is not?
Why is graphite used for making dry cell electrodes but diamond is not?
Give the general formula of an alkyne.
Give the general formula of an alkene.
Give the general formula of an alkane
Classify the following compounds as alkanes :
C2H4, C3H4, C4H8, C5H12, C5H8, C3H8, C6H6
Classify the following compounds as alkynes:
C2H4, C3H4, C4H8, C5H12, C5H8, C3H8, C6H6
Classify the following compounds as alkenes:
C2H4, C3H4, C4H8, C5H12, C5H8, C3H8, C6H6
Friedrich Wohler converted an inorganic compound into an organic compound in the laboratory.
Give the name and formula of inorganic compound.
Friedrich Wohler converted an inorganic compound into an organic compound in the laboratory.
Write the name and formula of organic compound formed.
Give the molecular formula of butane and mention the names of its two isomers. Name one fuel which contains both these isomers.
Give IUPAC names and formulae of an organic compound containing single bonds and the other containing a triple bond.
Which of the following is the molecular formula of benzene?
C6H6, C6H10, C6H12, C6H14
Which of the two has a branched chain : isobutane or normal butane?
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
How can diamonds be made artificially?
How do synthetic diamonds differ from natural ones?
Give any two differences between the properties of diamond and graphite. What causes these differences?
Why does the element carbon from a large number of carbon compounds?
Write down the structures and names of two isomers of butane (C4H10)
Give the name and structural formula of one member each of the following:
cycloalkane
Give the name and structural formula of one member each of the following:
alkyne
Give the name and structural formula of one member each of the following:
alkene
Give the name and structural formula of one member each of the following:
alkane
Give the common name of ethene.
Give the common name of ethyne.
Write the molecular formula and structure of benzene.
What is the unique property of carbon atom? How is this property helpful to us?
Explain why, diamond is hard while graphite is soft (though both are made of carbon atoms).
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethene
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
ethyne
Giving their structures, state the number of single bonds, double bonds and triple bonds (if any) in the following compounds:
benzene
Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in a molecule of cyclohexane?
Write two points of difference in the structures of diamond and graphite.
Explain why, graphite can be used as a lubricant but diamond cannot.
Explain why, diamond can be used in rock drilling equipment but graphite cannot.
State one use of diamond which depends on its 'extraordinary brilliance' and one use of graphite which depends on its being 'black and quite soft'.
What is diamond? Of what substance is diamond made?
Describe the structure of diamond. Draw a simple diagram to show the arrangement of carbon atoms in diamond.
Explain why, diamond has a high melting point.
State any two uses of diamond.
What is graphite?
what substance is graphite made?
Describe the structure of graphite with the help of a labelled diagram.
Why is graphite a good conductor of electricity but diamond is a non-conductor of electricity?
State any two uses of graphite.
Explain the term 'isomers'. Give one example of isomers.
Write electron-dot structure, of any one isomer of n-heptane (C7H16).
Write structural formula.
Write IUPAC name of the compound having the formula n-C4H10.
Give the IUPAC names for the following:
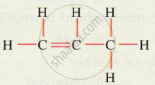
Give the IUPAC names for the given
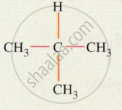
Give the IUPAC names for the following:
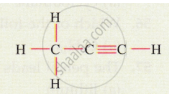
Give the IUPAC names for the following:
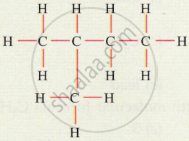
What are hydrocarbons? Explain with examples.
Explain the meaning of saturated and unsaturated hydrocarbons with two examples each.
Give the names and structural formulae of one saturated cyclic hydrocarbon and one unsaturated cyclic hydrocarbon.
Give one example of a hydrocarbon, other than pentane, having more than three isomers.
How many isomers of the following hydrocharbons are possible?
C4H10
How many isomers of the following hydrocharbons are possible?
C6H14
How many isomers of the following hydrocharbons are possible?
C3H8
How many isomers of the following hydrocharbons are possible?
C5H12
Buckminsterfullerene is an allotropic form of the element:
(a) phoshorus
(b) fluorine
(c) carbon
(d) sulphur
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6
(b) C6H12 and C5H12
(c) C4H6 and C6H12
(d) C2H6 and C4H10
The number of covalent bonds in pentane (molecular formula C5H12) is:
5
12
17
16
The property of self-combination of the atoms of the same element to form long chains is known as:
(a) protonation
(b) carbonation
(c) coronation
(d) catenation
A cyclic hydrocarbon having carbon-carbon single bonds as well as carbon-carbon double bonds in its molecule is:
(a) C6H12
(b) C6H14
(c) C6H6
(d) C6H10
The hydrocarbon 2-methylbutane is an isomer of:
(a) n-pentane
(b) n-butane
(c) propane
(d) iso-butane
An unsaturated hydrocarbon having a triple covalent bond has 50 hydrogen atoms in its molecule. The number of carbon atoms in its molecule will be:
(a) 24
(b) 25
(c) 26
(d) 28
An alkyne has seventy five carbon atoms in its molecule. The number of hydrogen atoms in its molecule will be:
(a) 150
(b) 148
(c) 152
(d) 146
A diamond-toothed saw is usually used for cutting:
(a) steel girders
(b) logs of wood
(c) marble slabs
(d) asbestos sheets
The organic compound prepared by Wohler from an inorganic compound called ammonium cyanate was:
(a) glucose
(b) urea
(c) uric acid
(d) vinegar
One of the following is not an allotrope of carbon. This is:
(a) diamond
(b) graphite
(c) cumene
(d) buckministerfullerene
The number of carbon atoms in the organic compound named as 2,2-dimethylpropane is:
(a) two
(b) five
(c) three
(d) four
The pair of elements which exhibits the property of catenation is:
sodium and silicon
chlorine and carbon
carbon and sodium
silicon and carbon
A saturated hydrocarbon has fifty hydrogen atom in its molecule. The number of carbon atoms in its molecule will be
(a) twenty five
(b) twenty four
(c) twenty six
(d) twenty seven
A hydrocarbon having one double bond has 100 carbon atoms in its molecule. The number of hydrogen atoms in its molecule will be
(a) 200
(b) 198
(c) 202
(d) 196
The hydrocarbon which has alternate single and double bonds arranged in the form of a ring is:
(a) cyclobutane
(b) benzene
(c) butene
(d) hexene
Which of the following cannot exhibit isomerism?
(a) C4H10
(b) C5H12
(c) C3H8
(d) C6H14
The pencil leads are made of mainly:
(a) lithium
(b) charcoal
(c) lead
(d) graphite
The number of isomers formed by the hydrocarbon with molecular formula C5H12 is:
(a) 2
(b) 5
(c) 3
(d) 4
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) fifty
(b) sixty
(c) seventy
(d) ninety
A solid element X has four electrons in the outermost shell of its atom. An allotrope Y of this element is used as a dry lubricant in machinery and also in making pencil leads.
(a) What is element X?
(b) Name the allotrope Y.
(c) State whether allotrope Y is a good conductor or non-conductor of electricity.
(d) Name one use of allotrope Y (other than lubrication and pencil leads)
(e) Name two other allotropes of element X.
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if A is a cyclic compound
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
if B is an open chain compound
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains single bonds as well as a double bond?
Two organic compounds A and B have the same molecular formula C6H12. Write the names and structural formulae:
Which compound contains only single bonds?
The solid element A exhibits the property of catenation. It is also present in the form of a gas B in the air which is utilised by plants in photosynthesis. An allotrope C of this element is used in glass cutters.
(a) What is element A?
(b) What is the gas B?
(c) Name the allotrope C.
(d) State another use of allotrope C (other than in glass cutters).
(e) Name another allotrope of element A which exists as spherical molecules.
(f) Name a yet another allotrope of element A which conduct electricity.
An element E exists in three allotropic forms A, B and C. In allotrope A, the atoms of element E are joined to form spherical molecules. In allotrope B, each atom of element E is surrounded by three other E atoms to form a sheet like structure. In allotrope C, each atom of element E is surrounded by four other E atoms to form a rigid structure.
(a) Name the element E.
(b) What is allotrope A.
(c) What is allotrope B?
(d) What is allotrope C?
(e) Which allotrope is used in making jewellery?
(f) Which allotrope is used in making anode of a dry cell?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which formula represent cyclohexane as well as hexene?
You are given the following molecular formulae of some hydrocarbons:
Which formula represents benzene?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae represent open chain unsaturated hydrocarbons having double bonds?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which two formulae represent unsaturated hydrocarbons having triple bonds?
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which three formulae can represent cyclic hydrocarbons?
Which of the following compounds can have a triple bond?
C2H4, C3H4, C3H6
Write the molecular and structural formula of a cyclic hydrocarbon whose molecule contains 8 atoms of carbon.
What is the molecular formula and structural formula of a cyclic hydrocarbon whose one molecule contains 8 hydrogen atoms?
Write the molecular formula of an alkane having 20 carbon atoms?
Write the molecular formula of an alkene having 20 carbon atoms?
Write the molecular formula of an alkyne having 20 carbon atoms?
Which of the following compounds can have a double bond?
C4H10; C5H8; C5H10
Which of the following hydrocarbons is unsaturated?
C3H4; C2H6
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 4 Carbon And Its Compounds Exercise 2 [Pages 239 - 243]
Write the molecular formula of ethanol.
What is the next higher homologue of methanol (CH3OH)?
Identify the functional group present in the following compound and name it according to IUPAC system:
CH3OH
Give the common name and IUPAC name of the simplest aldehyde.
What is the common name of methanal?
Write the names of the following functional group:

Write the names of the following functional group:
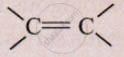
Name the simplest ketone.
What is the common name of propanone?
Write the IUPAC names of the following:
CH3COCH3
Write the IUPAC names of the following:
CH3COCH2CH3
Write the name and chemical formula of the simplest organic acid.
Write the IUPAC names, common names and formulae of the first two members of the homologous series of carboxylic acids.
What is the common name of methanoic acid.
What is the common name of ethanoic acid?
Draw the structure for the following compound.
Ethanoic acid
Draw the structures for the following compounds of Propanoic acid.
Give the common names and IUPAC names of the following compounds of HCOOH.
Give the common names and IUPAC names of the following compounds of CH3COOH.
Give the name and structural formula of one homologue of HCOOH.
Write the formulae of methanoic acid.
Write the formulae of ethanoic acid.
Give the common name and IUPAC name of C2H5OH.
Give the IUPAC name of the following compound:
C3H7OH
Give the name and structural formula of one member of the following:
Alcohols
Give IUPAC names of the following compounds:
C4H9OH
Give IUPAC names of the following compounds of C5H11OH
What is the common name of methanol?
What is the difference between two consecutive homologues:
(1) in terms of molecular mass?
(2) in terms of number and kind of atoms per molecule?
What type of fuels burn with a flame?
What type of fuels burn without a flame?
State whether the following statement is true of false:
The minimum number of carbon atoms in a ketone molecule is two.
Fill in the following blank with suitable word:
The next higher homologue of ethanol is ...............
Fill in the following blank with suitable word:
The next homologue of C2H5OH is ...............
Fill in the following blank with suitable word:
The next higher homologue of ethane is ...............
Fill in the following blank with suitable word.
The functional group present in ethanol is ...............
Organic compounds having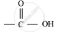 functional group are known as
functional group are known as
Give the general name of the class of compounds having the general formula CnH2n−2. Write name of the first member of this homologous series.
The general formula of a homologous series of carbon compounds if CnH2n. Write the molecular formula out the second and fourth members of the series.
Write the molecular formulae of the third and fifth members of homologous series of carbon compounds represented by the general formula CnH2n+2
Give the names and structural formulae of the next two higher homologues of methane.
The molecular formula of a hydrocarbon is C10H18. Name its homologous series.
Select the hydrocarbons which are members of the same homologous series. Give the name of each series.
C5H10; C3H8; C6H10; C4H10; C7H12; C8H16
Give the molecular formula of one homologue of the following:
C3H6
Give the molecular formula of one homologue of each of the following:
C2H6
By how many carbon atoms and hydrogen atoms do any two adjacent homologues differ?
Write the formula of the functional group present in carboxylic acids.
Name the functional group present in CH3—C ≡ CH.
Name the functional groups present in the following compound:
CH3CH2CH2OH
Name the functional groups present in the following compound:
CH3COCH3
Name the functional groups present in the following compound:
CH3CH2COOH
Name the functional groups present in the following compound:
CH3CHO
Write the IUPAC name and common name of CH3CI.
Draw the structure of chlorobutane.
Draw the structure for bromopentane. Are structural isomers possible for bromopentane?
Write the name and formula of an organic compound containing a ketone functional group.
Write the names and formulae for the first three members of the homologous series for chloroalkanes.
How would you name the following compound ?
CH3—CH2—Br
What is the general name of the organic compounds containing the -CO- group?
Which of the following compounds contains a carboxylic acid group?
CH3OH, CH3COOH, CH3CHO, CH3COCH3
How would you name the following compound?
Figure
Define a homologous series. Give the name and structural formula of one homologue of the following:
CH3OH
Write the molecular formula of the third member of the homologous series of carbon compounds with general formula CnHO2n+1OH.
Name any two fossil fuels.
Draw the structures for Butanone
Draw the structures for the following compounds of Propanone.
Write the IUPAC names of the following:
HCHO
Write the IUPAC names of the following:
CH3CHO
Write the IUPAC names of the following:
CH3CH2CHO
Write the IUPAC names of the following:
CH3CH2CH2CHO
Which functional group is likely to be present in an organic compound having the molecular formula C4H10O? Write the formula of the organic compound.
Match the formulae in group A with appropriate names from group B:
Group A: CH3COOH, CH3CHO, CH3OH
Group B: Ethanol, Methanol, Ethanal, Ethanoic acid
Draw the structure of butanoic acid.
What is the IUPAC name of acetic acid?
Which functional group do you think can be present in an organic compound having the molecular formula C5H10O2? Write the formula of the organic compound.
Give one example of the compound having the following functional group:
Carboxylic acid group
Give one example of the compound having the following functional group:
Halo group
Give one example of the compound having the following functional group:
Aldehyde group
Give one exampleof the compound having the following functional group:
Alcohol group
Give one example of the compound having the following functional group:
Alkene group
Give one example of the compound having the following functional group:
Alkyne group
What is the molecular formula and structure of the alcohol which can be thought to be derived from pentane?
Write the names of the following functional groups:

Write the name of the following functional group:

Write the name of the following functional group:

Write the names of the following functional groups:

Write the name of the following functional group:
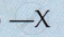
What makes the candle flame yellow and luminous?
What is a homologous series? Explain with an example.
State any four characteristics of a homologous series
The molecular formula of an organic compound is C18H36. Name its homologous series.
Select the hydrocarbons which are members of the same homologous series. Give the name of each series.
C5H10; C3H8; C6H10; C4H10; C7H12; C8H16
What is meant by 'heteroatom'? Give examples. Write the names and formulae of two organic compounds containing different heteroatoms.
What is meant by a functional group? Explain with an example.
Write three common functional groups present in organic compounds. Giver their symbols/formulae.
Name the functional groups present in the following compound:
CH3COCH2CH3
Name the functional groups present in the following compound:
CH3COOH
Name the functional groups present in the following compound.
C2H5OH
Name the functional groups present in the following compound:
CH3CH2CHO
Name the functional group which always occurs in the middle of a carbon chain.
Draw the structures for the following compounds:
Ethanal
Draw the structures for the following compounds:
Butanal
Draw the structures for the following compounds of Pentanal
Draw the structures for the following compounds:
Propanal
What happens when carbon burns in air? Write the chemical equation of the reaction which takes place.
Why are coal and petroleum called fossil fuels?
Explain how coal was formed in the earth.
Describe how petroleum was formed in the earth.
Name a fossil fuel other than coal and petroleum.
The molecular formula of a homologue of butane is:
(a) C4H8
(b) C3H6
(c) C4H6
(d) C3H8
One of the following molecular formula can represent two organic compounds having different functional groups. This molecular formula is:
(a) C5H12O
(b) C5H10O
(c) C5H10O2
(d) C5H12
The number of carbon atoms present in the molecule of fifth member of the homologous series of alkynes is:
(a) four
(b) five
(c) six
(d) seven
One of the following burns without producing a flame. This is:
(a) wood
(b) charcoal
(c) LPG
(d) candle
The functional group which always occurs in the middle of a carbon chain is:
(a) alcohol group
(b) aldehyde group
(c) carboxyl group
(d) ketone group
The molecular formulae of some organic compounds are given below. Which of these compounds contains an aldehyde group?
(a) C3H8O
(b) C3H6O2
(c) C3H6O
(d) C3H7Cl
The organic compounds which are isomeric with one another are:
(a) alcohols and aldehydes
(b) aldehydes and carboxylic acids
(c) ketones and aldehydes
(d) alcohols and ketones
The fuel which usually burns with a blue flame is:
(a) coal
(b) LPG
(c) candle wax
(d) kerosene (in lamp)
Which of the following burns by producing a yellow, luminous flame?
(a) natural gas
(b) coke
(c) wax
(d) charcoal
The molecular formula of an organic compound is C48H94. This compound belongs to the homologous series of:
(a) alkenes
(b) aldehydes
(c) alkynes
(d) alkanes
One of the following molecular formulae represents a ketone. This formula is:
(a) C5H12O
(b) C6H12O2
(c) C6H14O
(d) C6H12O
Which one of the following is not a fossil fuel?
(a) petrol
(b) cock
(c) charcoal
(d) coal
Butanone is a four-carbon compound having the functional group:
(a) −OH
(b) −COOH
(c) −CO−
(d) −CHO
The molecular formula of the third member of the homologous series of ketones is:
(a) C4H8O
(b) C3H6O
(c) C5H10O
(d) C6H12O
The functional group present in propanal is:
(a) −OH
(b) −COOH
(c) −CO−
(d) −CHO
An organic compound having the molecular formula C3H6O can exist in the form of two isomers A and B having different functional groups. The isomer A is a liquid which is used as a solvent for nail polish. The isomer B is also a liquid. An aqueous solution of one of the lower homologues of B is used for preserving biological specimens in the laboratory
(a) What is compound A?
(b) Write the electron-dot structure of A.
(c) What is compound B?
(d) Write the electron-dot structure of B.
(e) Name the lower homologue of compound B which is used in preserving biological specimens.
A hard material X which is mined from the earth is used as a house hold fuel and also for the generation of electricity at Thermal Power Stations. A soft material Y is also used us a fuel in the form of candles. A gaseous material Z which occurs along with petroleum is increasingly being used as a fuel in running vehicles in its compressed form.
(a) What are materials, X, Y and Z?
(b) When materials X, Y and Z are burned separately:
(1) When material burns by producing a yellow, luminous flame?
(2) Which material ultimately burns without producing a flame?
(3) Which material can burn in a gas stove by producing a blue flame?
Three organic compounds A, B and C have the following molecular formulae: C4H8O2
Which compound contains an alcohol group? Write its name and structural formula.
Three organic compounds A, B and C have the following molecular formulae: C4H10O
Which compound contains a carboxyl group? Write its name and structural formula.
Three organic compounds A, B and C have the following molecular formulae: C4H8O
Which molecular formula can represent an aldehyde as well as a ketone? Write the names and structural formulae of the aldehyde and ketone represented by this molecular formula.
A colourless organic liquid X of molecular formula C2H4O2 turns blue litmus to red. Another colourless organic liquid Y of molecular formula C3H6O has no action on any litmus but it is used as a nail polish remover. A yet another colourless organic liquid Z of molecular formula C2H6O has also no action on litmus but it is used in tincture of iodine.
(a) Name the liquid X. To which homologous series does it belong? Give the name of another member of this homologous series.
(b) Name the liquid Y. To which homologous series does it belong? Write the name of another member of this homologous series.
(c) Can you name an organic compound having the same molecular formula as liquid Y but which belongs to a different homologous series? What is this homologous series?
(d) Name the liquid Z. To which homologous series does it belong? Write the name of another member of this homologous series.
You are given an organic compound having the molecular formula C3H8. Give the name and formula of the compound formed:
when one H atom of C3H8 is replaced by a Cl atom.
You are given an organic compound having the molecular formula C3H8. Give the name and formula of the compound formed:
when one H atom of C3H8 is replaced by a OH group.
You are given an organic compound having the molecular formula C3H8. Give the name and formula of the compound formed:
when one H atom of C3H8 is replaced by a CHO group.
You are given an organic compound having the molecular formula C3H8. Give the name and formula of the compound formed
when one H atom of C3H8 is replaced by a COOH group.
You are given an organic compound having the molecular formula C3H8. Give the name and formula of the compound formed:
when two H atoms joined to the middle carbon atom of C3H8 are replaced by one O atom.
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 4 Carbon And Its Compounds Exercise 3 [Pages 262 - 266]
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Which of the following will give brisk effervescence with sodium hydrogen carbonate and why?
CH3COOH, CH3CH2OH
Name the functional group present in an organic compound which gives brisk effervescence with NaHCO3.
Name the hydrocarbon formed when ethanol is heated with conc. H2SO4 at 170°C? What is this reaction known as?
Why does ethyne (acetylene) burns with a sooty flame?
Name the product formed when hydrogen is added to ethene.
Explain why, ethene decolourises bromine water whereas ethane does not.
Name two catalysts which can be used in the hydrogenation of unsaturated compounds.
State two disadvantages of incomplete combustion.
What happens when (give chemical equation):
Sodium reacts with ethanol (ethyl alcohol)
Describe one reaction of ethanol.
Name one liquid carbon compound which is being used as an additive in petrol in some countries.
What are the raw materials required for making soap in a laboratory (or at home)?
Would you be able to check if water is hard by using a detergent?
Describe a test for carboxylic acid.
Why is the conversion of ethanol into ethanoic acid an oxidation reaction?
Explain why, alkanes are excellent fuel.
Name one chemical compound which can be used to distinguish between ethanol and ethanoic acid.
Complete the following equation:
`CH_3 CH_2 OH` 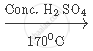
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 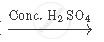
Complete and balance the following equation:
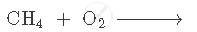
Complete and balance the following equation:
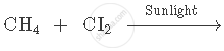
Fill in the following blank with suitable word:
The process of burning of a hydrocarbon in the presence of air to give CO2, H2O, heat and light is known as .............
Fill in the following blank with suitable word:
The sodium salt of a long chain fatty acid is called .............
Fill in the following blank with suitable word:
............. is better than soap for washing clothes when the water is hard.
Fill in the following blank with a suitable word:
The organic acid present in vinegar is ______.
Which of the following hydrocarbons will give substitution reactions and why?
CH4, C3H6, C3H8, C4H6, C5H12, C5H10
Which of the following will give addition reaction and why?
C4H10, C2H6, C2H4, CH4, C3H8, C3H4
Write the chemical equation of the reaction which takes place during the burning of ethanol in air.
Why is ethanol used as a fuel?
State two used of ethanol (other than as a fuel).
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
What change will you observe if you test soap solution with a litmus paper (red and blue)? Give reason for your observation.
What is meant by denatured alcohol? What is the need to denature alcohol?
How would you test for an alcohol?
Give the harmful effects of drinking alcohol.
Explain why, methanol is much more dangerous to drink than ethanol.
How would you convert of thanol into ethene?
propanol into propanoic acid?
Name the process in each case and write the equations of the reactions involved.
Give reasons for the following observation.
Air holes of a gas burner have to be adjusted when the vessels being heated get blackened by the flame.
Use of synthetic detergents causes pollution of water.
What would be observed on adding a 5% alkaline potassium permanganate solution drop by drop to some warm ethanol in a test-tube? Write the name of the compound formed during the chemical reaction. Also write chemical equation of the reaction which takes place.
How would you distinguish experimentally between an alcohol and carboxylic acid on the basis of a chemical property?
Name the functional group of organic compounds that can be hydrogenated. With the help of a suitable example, explain the process of hydrogenation, mentioning the conditions of the reaction and any one change in physical property with the formation of the product. Name any one natural source of organic compounds that are hydrogenated.
Name the gas evolved when ethanol reacts with sodium.
What type of compound is formed when a carboxylic acid reacts with an alcohol in the presence of conc. H2SO4?
What will you observe when dilute ethanoic acid and dilute hydrochloric acid are put on universal indicator paper, one by one? What does it show?
What type to compound is CH3COOH?
What substance should be oxidised to prepare CH3COOH?
What is the physical state of CH3COOH?
State one advantage of soaps over detergent.
What happens when ethanol reacts with ethanoic acid in the presence of a little of concentrated sulphuric acid? Write equation of the reaction involved.
What happens when ethanol is heated with concentrated sulphuric acid at 170°C? Write the equation of the reaction which takes place.
What happens when ethanol is oxidised with alkaline potassium permanganate (or acidified potassium dichromate)? Write the equation of the reaction involved.
Choose those compounds from the following which can turn blue litmus solution red:
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO
Give reasons for your choice.
Explain the process of preparation of soap in laboratory.
Why is common salt (sodium chloride) added during the preparation of soap?
Why is soap not suitable for washing clothes when the water is hard?
What happens when methane (natural gas) burns in air? Write the chemical equation of the reaction involved.
What happens when ethanoic acid reacts with sodium carbonate? Write chemical equation of the reaction involved.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Describe, giving equation, a chemical reaction which is characteristic of saturated hydrocarbons (or alkanes).
What is an oxidising agent?
Name two oxidising agents which can oxidise ethanol to ethanoic acid.
What is a detergent? Name one detergent.
Describe one reaction of a carboxylic acid.
Write names and formulae of hydrocarbons containing a single and a double bond (one example for each). Give one characteristic chemical property of each.
Why have detergents replaced soap as a washing agent?
How does ethanoic acid react with sodium hydrogen carbonate? Give equation of the reaction which takes place.
Why are carbon and its compounds used as fuels for most applications?
Which of the two is better for washing clothes when the water is hard: soap or detergent? Give reason for your answer.
What is meant by a substitution reaction? Give an example (with equation) of the substitution reaction of an alkane.
How is soap made? Write a word equation involved in soap making.
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
How would you distinguish between ethanol and ethanoic acid by chemical test?
Explain the formation of scum when hard water is treated with soap.
What happens when methane reacts with chlorine? Give equation of the reaction which takes place.
Explain hydrogenation with the help of a chemical equation. State the role of this reaction in industry.
Give any two differences between soaps and detergents.
What happens when ethanoic acid reacts with sodium hydroxide? Write equation of the reaction involved.
What happens when vegetable oils are hydrogenated? Name the catalyst used.
What is the advantage of detergents over soaps for washing clothes? Also state one disadvantage.
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
A mixture of ethyne (acetylene) and oxygen is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Name a chemical reaction which is characteristic of unsaturated hydrocarbons (like alkenes and alkynes).
What is meant by an addition reaction? Give an example (with equation) of an addition reaction of an alkene.
What is added to groundnut oil when it is to be converted to vanaspati ghee?
Which of the two is better for our health : butter or vegetable oil? Why?
When ethanoic acid reacts with sodium hydrogen carbonate, then a salt X is formed and a gas Y is evolved. Name the salt X and gas. Y Describe an activity with the help of a labelled diagram of the apparatus used to prove that the evolved gas is the one which you have named. Also write the chemical equation of the reaction involved.
Give any two uses of ethanoic acid.
Esters are sweet-smelling substances and are used in making perfumes. Describe an activity for the preparation of an ester with the help of a well labelled diagram. Write an equation for the chemical reaction involved in the formation of the ester. Also write the names of all the substances involved in the process of esterification.
State any two uses of esters.
Name the reaction which is usually used in the conversion of vegetables oils to fats. Explain the reaction involved in detail. Write a chemical equation to illustrate your answer.
What is saponification? Write the chemical equation of the reaction involved in this process. Name all the substances which take part in this process and also those which are formed.
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
What is a soap? Name one soap.
Describe the structure of a soap molecule with the help of a diagram.
Explain the cleansing action of soap. Draw diagrams to illustrate your answer.
While cooking, if the bottom of the utensil is getting blackened on the outside, it means that:
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
When ethanol is heated with alkaline potassium permanganate solution, it gets converted into ethanoic acid. In this reaction, alkaline potassium permanganate acts as:
(a) reducing agent
(b) oxidising agent
(c) catalyst
(d) dehydrating agent
When ethanol is heated with concentrated sulphuric acid at 170°C, it gets converted into ethene. In this reaction, concentrated sulphuric acid acts as:
(a) oxidising agent
(b) catalyst
(c) dehydrating agent
(d) reducing agent
When a vegetable oil is treated with hydrogen in the presence of nickel (or palladium) catalyst, it forms a fat. This is an example of:
(a) anodising reaction
(b) substitution reaction
(c) displacement reaction
(d) addition reaction
The soap molecule has a ______.
hydrophilic head and a hydrophobic tail
hydrophobic head and a hydrophilic tail
hydrophobic head and a hydrophobic tail
hydrophilic head and a hydrophilic tail
Chlorine reacts with saturated hydrocarbons at room temperature in the:
(a) absence of sunlight
(b) presence of sunlight
(c) absence of moisture
(d) presence of H2SO4
In a soap micelle, the soap molecules are arranged radially with:
(a) ionic ends directed towards the centre and hydrocarbon ends directed outwards
(b) hydrocarbon ends directed towards the centre and ionic ends directed outwards
(c) both ionic ends and hydrocarbon ends directed toward the centre
(d) both hydrocarbon ends and ionic ends directed outwards
When ethanol reacts with sodium metal, it forms two products. These products are:
(a) sodium ethanaoate and oxygen
(b) sodium ethanaoate and hydrogen
(c) sodium ethoxide and oxygen
(d) sodium ethoxide and hydrogen
Vinegar is a solution of about:
(a) 5 to 8 per cent ethanoic acid in alcohol
(b) 5 to 8 per cent ethanoic acid in water
(c) 50 to 80 per cent ethanoic acid in water
(d) 50 to 80 per cent ethanoic acid in alcohol
One of the following substances is not added to make denatured alcohol. This is:
(a) methyl alcohol
(b) copper sulphate
(c) chloroform
(d) pyridine
One of the following organic compounds cannot decolourise the red-brown colour bromine water. This compound is:
(a) C14H28
(b) C14H28
(c) C14H28
(d) C14H28
The substance which can produce brisk effervescence with baking soda solution is:
(a) ethanol
(b) vegetable oil
(c) vinegar
(d) soap solution(b) hydrocarbon ends directed towards the centre and ionic ends directed outwards
In a soap micelle, the soap molecules are arranged radially with the hydrocarbon ends, i.e. hydrophobic, directed towards the centre; and, ionic ends, i.e. hydrophilic, directed outwards.
The chemical which is not required for the preparation of soap in the laboratory is:
(a) vegetable oil
(b) baking soda
(c) caustic soda
(d) common salt
Which of the following can damage optic nerve leading to blindness, if taken internally?
(a) CH3COOH
(b) C2H5OH
(c) NaHCO3
(d) CH3OH
The usual disease caused by the excessive drinking of alcohol over a long period of time is:
(a) diabetes
(b) cataract
(c) cirrhosis
(d) arthritis
Which of the following molecular formula corresponds to ethylbutanoate ester?
(a) C5H10O2
(b) C6H12O2
(c) C7H14O2
(d) C8H16O2
A neutral organic compound X of molecular formula C2H6O on oxidation with acidified potassium dichromate gives an acidic compound Y. Compound X reacts with Y on warming in the presence of conc. H2SO4 to give a sweet smelling substance Z. What are X, Y and Z?
Consider the following organic compounds:
HCHO, C2H5OH, C2H6, CH3COOH, C2H5CI
Choose two compounds which can react in the presence of conc. H2SO4 to form an ester. Give the name and formula of the ester formed.
A neutral organic compound is warmed with some ethanoic acid and a little of conc. H2SO4. Vapours having sweet smell (fruity smell) are evolved. What type of functional group is present in this organic compound?
The structural formula of an ester is :

Write the formula of the acid and the alcohol from which it is formed.
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is most likely to be sweet-smelling?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound on treatment with conc. H2SO4 at 170°C forms an alkene?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound on repeated chlorination forms chloroform?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is added to alcohol to denature it?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is a constituent of vinegar?(e) Which compound is a constituent of vinegar?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is used to sterilise wounds and syringes?
An organic acid X is a liquid, which often freezes during winter time in cold countries, having the molecular formula C2H4O2. On warming it with methanol in the presence of a few drops of concentrated sulphuric acid, a compound Y with a sweet smell is formed.
(a) Identify X and Y. Also write their formulae showing the functional group present in them.
(b) Write a chemical equation for the reaction involved.
An organic compound A having the molecular formula C3H8O is a liquid at room temperature. The organic liquid A reacts with sodium metal to evolve a gas which burns causing a little explosion. When the organic liquid A heated with concentrated sulphuric acid at 170°C, it forms a compound B which decolourizes bromine water. The compound B adds on one molecule of hydrogen in the presence of Ni as catalyst to forms compound C which gives substitution reactions with chlorine.
(a) What is compound A?
(b) What is compound B?
(c) What type of reaction occurs when A is converted into B?
(d) What is compound C?
(e) What type of reaction takes place when B is converted into C?
An organic compound A (molecular formula C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H6O2). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and
Which of the following hydrocarbons can decolourise bromine water and which cannot? Why?
C6H12, C6H14, C6H10
A four carbons atoms containing neutral organic compound X reacts with sodium metal to evolve a gas which burns with 'pop' sound. Another four carbon atoms containing carbon compound reacts with sodium hydrogen carbonate to evolve a gas which turns lime water milky. When compounds X and Y are heated together in the presence of a little of concentrated sulphuric acid, then a new compound Z is formed.
(a) What is compound X ? Also write its formula.
(b) What is compound Y ? Also write its formula.
(c) What is compound Z ? Also write its formula.
(d) What type of smell is given by compound Z?
(e) What is the general name of compounds like Z?
(f) What is the general name of the reaction which which takes place between X and Y to form Z?
Solutions for 4: Carbon And Its Compounds
![Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 4 - Carbon And Its Compounds Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 4 - Carbon And Its Compounds - Shaalaa.com](/images/9352831810-chemistry-science-english-class-10_6:7eeaf5db0f4a49c580d3c8746ff5ab36.jpg)
Lakhmir Singh solutions for Chemistry (Science) [English] Class 10 chapter 4 - Carbon And Its Compounds
Shaalaa.com has the CBSE Mathematics Chemistry (Science) [English] Class 10 CBSE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Lakhmir Singh solutions for Mathematics Chemistry (Science) [English] Class 10 CBSE 4 (Carbon And Its Compounds) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Lakhmir Singh textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry (Science) [English] Class 10 chapter 4 Carbon And Its Compounds are Carbon: a Versatile Element, The Covalent Bond, Saturated and Unsaturated Carbon Compounds, Chains, Branches and Rings of Carbon Compound, Homologous Series of Carbon Compound, Ethanol, Ethanoic Acid, Soap, Properties of Carbon, Nomenclature of Organic Compounds, Allotropy and Allotropes of Carbon, Crystalline Allotropes of Carbon: Diamond, Crystalline Allotropes of Carbon: Graphite, Crystalline Allotropes of Carbon: Fullerene, Functional Groups in Carbon Compounds, Detergents, Cleansing Action of Soap.
Using Lakhmir Singh Chemistry (Science) [English] Class 10 solutions Carbon And Its Compounds exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Lakhmir Singh Solutions are essential questions that can be asked in the final exam. Maximum CBSE Chemistry (Science) [English] Class 10 students prefer Lakhmir Singh Textbook Solutions to score more in exams.
Get the free view of Chapter 4, Carbon And Its Compounds Chemistry (Science) [English] Class 10 additional questions for Mathematics Chemistry (Science) [English] Class 10 CBSE, and you can use Shaalaa.com to keep it handy for your exam preparation.
