Advertisements
Advertisements
Question
Name two oxidising agents which can oxidise ethanol to ethanoic acid.
Solution
Two oxidising agents which can oxidise ethanol to ethanoic acid are alkaline potassium permanganate (KMnO4) and acidified potassium dichromate (K2Cr2O7).
APPEARS IN
RELATED QUESTIONS
In an experiment to study the properties of ethanoic acid, a student takes about 3 mL of ethanoic acid in a dry test tube. He adds an equal amount of distilled water to it and shakes the test tube well. After some time he is likely to observe that
(A) a colloid is formed in the test tube.
(B) the ethanoic acid dissolves readily in water.
(C) the solution becomes light orange.
(D) water floats over the surface of ethanoic acid.
While studying saponification reaction, a student measures the temperature of the reaction mixture and also finds its nature using blue/red litmus paper. On the basis of his observations the correct conclusion would be
(A) the reaction is exothermic and the reaction mixture is acidic.
(B) the reaction is endothermic and the reaction mixture is acidic.
(C) the reaction is endothermic and the reaction mixture is basic.
(D) the reaction is exothermic and the reaction mixture is basic.
When you add about 2 ml of acetic acid to a test tube containing an equal amount of distilled water and leave the test tube to settle after shaking its contents, what will you observe in the test tube after about 5 minutes?
(A) A white precipitate settling at its bottom
(B) A clear colourless solution
(C) A layer of water over the layer of acetic acid
(D) A layer of acetic acid over the layer of water
When zinc powder is added to acetic acid ______________
(a) the mixture becomes warm
(b) a gas is evolved
(c) the colour of the mixture becomes yellow
(d) a solid settles at the bottom
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 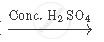
A student takes Na2CO3 powder in a test tube and pours some drops of acetic acid over it. He observes:
(A) no reaction in the test tube
(B) colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) white fumes with smell of vinegar
How Will You Carry Out the Following Conversions?
Ehane to acitic acid
Choose the correct answer:
Select the acid which contains four hydrozen atoms in it.
Give the balanced chemical equation of the following reaction:
Evolution of carbon dioxide by the action of ethanoic acid with NaHCO3.
Ester is formed by the reaction between ______.
