Advertisements
Advertisements
Question
Fill in the following blank with suitable word:
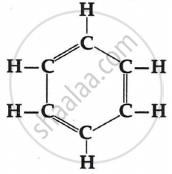
The property of carbon atoms to form long chains in compounds is called ...........
Solution
The property of carbon atoms to form long chains in compounds is called catenation.
APPEARS IN
RELATED QUESTIONS
Draw the structures for Bromopentane*
Fill in the following blank with suitable word:
Compounds of carbon with hydrogen alone are called ..........
Explain the meaning of saturated and unsaturated hydrocarbons with two examples each.
The pair of elements which exhibits the property of catenation is:
The functional group which always occurs in the middle of a carbon chain is:
(a) alcohol group
(b) aldehyde group
(c) carboxyl group
(d) ketone group
Which of the following are correct structural isomers of butane?
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}\backslash\phantom{..}|\\
\phantom{.....}\ce{H}\phantom{.......}\ce{C - H}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{....}\phantom{....}|\\
\ce{H}\phantom{........}\ce{H}\\
|\\
\ce{H - C - H}\\
|\\\ce{H}
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}
\end{array}\]
How many isomers are possible for the compound with the molecular formula C4H8? Draw the electron dot structure of branched-chain isomer.
Draw two structural isomers of butane.
Draw the structure of propanol.
Name the following: