Advertisements
Advertisements
Question
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many hexagons of carbon atoms are present in one molecule of buckminsterfullerene?
Solution
In one molecule of buckminsterfullerene, 20 hexagons of carbon atoms are present.
APPEARS IN
RELATED QUESTIONS
Ethane, with the molecular formula C2H6 has ______.
Name the black substance of pencil. Will the current flow through the electrical circuit when we use the sharpened ends of the pencil to complete the circuit?
How does graphite act as a lubricant?
Fill in the blank in the following sentence:
Two chlorine atoms combine to form a molecule. The bond between them is known as ..........
Give two general properties of ionic compounds and two those of covalent compounds.
What type of bonding would you expect between Carbon and Chlorine?
Draw the electron-dot structure of N2 and state the type of bonding.
Why are covalent compounds generally poor conductors of electricity?
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
A covalent molecule having a double bond between its atoms is:
(a) Hydrogen
(b) Oxygen
(c) water
(d) ammonia
A hydrocarbon having one double bond has 100 carbon atoms in its molecule. The number of hydrogen atoms in its molecule will be
(a) 200
(b) 198
(c) 202
(d) 196
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) fifty
(b) sixty
(c) seventy
(d) ninety
Define a coordinate bond and give the conditions for its formation.
Give reason as to why hydrogen chloride can be termed as a polar covalent compound.
Acids dissolve in water and produce positively charged ions. Draw the structure of these positive ions.
The molecular masses of a carbon compound spread over a range of _______.
Write an Explanation.
Alkene
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc} \phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\ \phantom{..}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C- C- H}\\ \phantom{.}|\phantom{....}|\phantom{....}|\\ \ce{H - C - H}\\ |\\\ce{H}\end{array}\] |
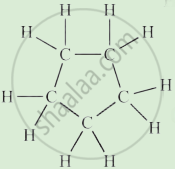
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
- Identify which element(s) will form covalent bonds with carbon.
- “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
