Advertisements
Advertisements
प्रश्न
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many hexagons of carbon atoms are present in one molecule of buckminsterfullerene?
उत्तर
In one molecule of buckminsterfullerene, 20 hexagons of carbon atoms are present.
APPEARS IN
संबंधित प्रश्न
Write any three features and give two examples of covalent compounds
Name the black substance of pencil. Will the current flow through the electrical circuit when we use the sharpened ends of the pencil to complete the circuit?
Fill in the following blank with suitable word:
The form of carbon which is known as black lead is ...........
State one test by which sodium chloride can be distinguished from sugar.
One of the following compounds is not ionic in nature. This compound is:
(a) Lithium chloride
(b) Ammonium chloride
(c) Calcium chloride
(d) Carbon tetrachloride
What is the difference between ionic compounds and polar covalent compounds?
Define a coordinate bond and give the conditions for its formation.
Give two example in following case:
Liquid non polar compounds
Explain the following briefly:
Sodium chloride dissolves in water but carbon tetra chloride is insoluble in water.
The following table shows the electronic configuration of the elements W, X, Y, Z:
|
Element |
W |
X |
Y |
Z |
|
Electronic |
2,8,1 |
2,8,7 |
2,5 |
1 |
Answer the following questions based on the table above:
What type of bond is formed between Y and Z.
State the type of bonding in the following molecule.
Water
Draw the electron dot diagram and structure of :
methane
State the type of bond formed when the combining atom has small E.N. difference.
Element A has 2 electrons in its M shell. Element B has atomic number 7.
If B is a diatomic gas, write the equation for the direct combination of A and B to form a compound.
Give an example of the covalent bond formed by
(i) Similar atoms (ii) Dissimilar atoms
Name two carbon compounds used in day-to-day life.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
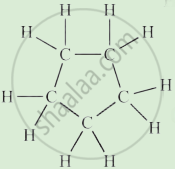 |
Cation is formed when ______.
Which of the following statements are correct for carbon compounds?
- Most carbon compounds are good conductors of electricity.
- Most carbon compounds are poor conductors of electricity.
- Force of attraction between molecules of carbon compounds is not very strong.
- Force of attraction between molecules of carbon compounds is very strong.
