Advertisements
Advertisements
Question
Give two general properties of ionic compounds and two those of covalent compounds.
Solution
| Ionic Compounds | Covalent Compounds |
| Ionic compounds usually have high melting and boiling points. | Covalent compounds usually have low melting and boiling points. |
| Ionic compounds generally conduct electricity when dissolved in water or in a molten state. | Covalent compounds usually do not conduct electricity. |
APPEARS IN
RELATED QUESTIONS
What are covalent compounds?
List three characteristic properties of covalent compounds.
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Choose the most appropriate answer from the following list of oxides which fit the description.
A covalent oxide of a metalloid.
What type of chemical bond is formed between carbon and bromine?
What type of bonds are present in Cl2 molecule? Draw their electron-dot structures.
State one test by which sodium chloride can be distinguished from sugar.
Why are covalent compounds generally poor conductors of electricity?
Explain the following term with example.
Covalent bond
What is the term defined below?
A bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair.
Fill in the blank from the choice given in bracket.
The compound that does not have a lone pair of electrons is ___________. (Water, Ammonia, carbon tetrachloride)
Element A has 2 electrons in its M shell. Element B has atomic number 7.
Write equations to show how A and B form ions.
Name two carbon compounds used in day-to-day life.
From the following hydrocarbon _______ is the cyclic hydrocarbon.
The number of electrons in the valence shell of a carbon atom is 4.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
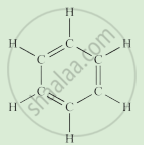 |
Give an example for each of the following statement
A compound in which two Covalent bonds are formed.
Give an example for each of the following statement.
Formation of coordinate covalent bond between NH3 ➝ BF3 molecules
State the reasons, why carbon cannot
- Lose four electrons to form C4+ cation and
- Gain four electrons to form C4- anion.
How does carbon overcome this problem to form compounds?
