Advertisements
Advertisements
Question
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Solution
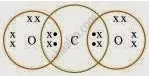
APPEARS IN
RELATED QUESTIONS
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – the eight atoms of sulphur are joined together in the form of a ring.)
State the type of bonding in the following molecule.
Water
Name the black substance of pencil. Will the current flow through the electrical circuit when we use the sharpened ends of the pencil to complete the circuit?
What do you call the compounds having the same molecular formula but different structural arrangements of atoms?
State one major difference between covalent and ionic bonds and give one example each of covalent and ionic compounds.
Give one example of a molecule containing a single covalent bond.
Why does carbon form compounds mainly by covalent bonding?
Draw the electron-dot structure of a hydrogen chloride molecule.
One of the following contains a double bond as well as single bonds. This is:
(a) CO2
(b) O2
(c) C2H4
(d) C2H2
A saturated hydrocarbon has fifty hydrogen atom in its molecule. The number of carbon atoms in its molecule will be
(a) twenty five
(b) twenty four
(c) twenty six
(d) twenty seven
The pencil leads are made of mainly:
(a) lithium
(b) charcoal
(c) lead
(d) graphite
What are the conditions necessary for the formation of covalent molecules?
State the type of bonding in the following molecule.
Hydroxyl ion
State the type of bonding in the following molecule.
Ammonium ion
Complete the following:
When the nuclei of two different reacting atoms are of ______ mass, then a bond so formed is called ______ covalent bond. (equal, unequal, polar, non-polar)
Give two example in following case:
Gaseous polar compounds
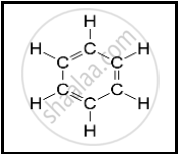
The following structural formula belongs to which carbon compound?
Explain the following briefly.
Pure water does not conduct electricity, but on adding sodium chloride to it, it starts conducting electricity.
Explain the following briefly:
Cl2 is a non polar molecule, while HCl is a polar molecule.
Give examples for the following:
Two gaseous non polar compounds.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
Explain the bonding in methane molecule using electron dot structure.
An element L consists of molecules.
Why L is heated with iron metal, it forms a compound FeL. What chemical term would you use to describe the change undergone by L?
The particles present in strong electrolytes are
State the type of bonding in the following molecule.
Water
Draw the electron dot diagram and structure of magnesium chloride.
State the type of bond formed when the combining atom has zero E.N. difference.
Name greenish-yellow gas which also bleaches.
Complete the following activity.
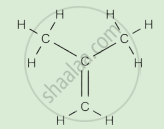
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
Give an example for each of the following statement.
A compound in which three covalent bonds are formed.
Identify the incorrect statement and correct them.
- Like covalent compounds, coordinate compounds also contain charged particles (ions). So they are good conductors of electricity.
- Ionic bond is a weak bond when compared to Hydrogen bond.
- Ionic or electrovalent bonds are formed by mutual sharing of electrons between atoms.
- Loss of electrons is called Oxidation and gain of electron is called Reduction.
- The electrons which are not involved in bonding are called valence electrons.
Discuss in brief about the properties of coordinate covalent compounds.
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules.
Which of the following is the formula of Butanoic acid?
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2, 6 |
| Q | 2, 8, 1 |
| R | 2, 8, 7 |
| S | 2, 8, 8 |
- Identify which element(s) will form covalent bonds with carbon.
- “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
What is a covalent bond?
"Carbon prefers to share its valence electrons with other atoms of carbon or with atoms of other elements rather than gaining or losing the valence electrons in order to attain noble gas configuration." Give reasons to justify this statement.
Name the following:
\[\ce{CH3 - CH2CH = CH2}\]
