Advertisements
Advertisements
Question
Draw the electron-dot structure of N2 and state the type of bonding.
Solution
Electron-dot structure of N2 is

In N2, one nitrogen atom shares its three electrons with another nitrogen atom to form a covalent bond.
APPEARS IN
RELATED QUESTIONS
What are covalent compounds?
Choose the most appropriate answer from the following list of oxides which fit the description.
A covalent oxide of a metalloid.
What is the atomic number of carbon. Write its electronic configuration.
Give two general properties of ionic compounds and two those of covalent compounds.
Draw the electron-dot structure of NH3 and state the type of bonding.
The electronic configurations of three elements X, Y and Z are as follows:
| X | 2, 4 |
| Y | 2, 7 |
| Z | 2, 1 |
(a) Which two elements will combine to form an ionic compound?
(b) Which two elements will react to form a covalent compound?
Give reasons for your choice.
Explain the following term with example.
Homopolymer
Write answer as directed.
What causes the existance of very large number of carbon compound ?
Choose the correct answer from the options given below
Condition favorable for formation of a covalent bond is
State the type of bond formed when the combining atom has small E.N. difference.
Covalent bonds can be single, double or triple covalent bonds. How many electrons are shared in each? Give an example of each type.
Explain covalent bond with example.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc}\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\ \phantom{.....}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C = C}\\\phantom{.....}|\phantom{.........}|\\ \phantom{.....}\ce{H}\phantom{........}\ce{H}\end{array}\] |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
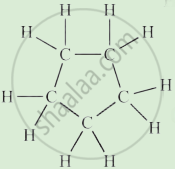 |
Consider the coordination compound, K2[Cu(CN)4]. A coordinate covalent bond exists between:
Which of the following is the purest form of carbon?
Mineral acids are stronger acids than carboxylic acids because
- mineral acids are completely ionised
- carboxylic acids are completely ionised
- mineral acids are partially ionised
- carboxylic acids are partially ionised
Molecular reactions are ______ in the covalent compound.
