Advertisements
Advertisements
Question
Explain covalent bond with example.
Answer in Brief
Solution
The chemical bond which is formed by sharing of two valence electrons between two atoms is called a covalent bond.
E.g. Formation of H2 molecule:
The atomic number of hydrogen is 1. It has 1 electron in its valence shell. It requires one more electron to complete its duplet and attain the stable electronic configuration of helium. Therefore, two hydrogen atoms share their electrons with each other to form H2 molecule. A single covalent bond is formed between two hydrogen atoms by sharing of two electrons (one pair of shared electrons).
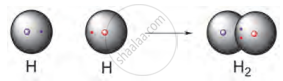
OR H - H
shaalaa.com
Is there an error in this question or solution?
