Advertisements
Online Mock Tests
Chapters
![SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC chapter 9 - Carbon compounds SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC chapter 9 - Carbon compounds - Shaalaa.com](/images/science-and-technology-1-english-10-standard-ssc_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 9: Carbon compounds
Below listed, you can find solutions for Chapter 9 of Maharashtra State Board SCERT Maharashtra for Science and Technology 1 [English] 10 Standard SSC.
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Choose the correct option.
Choose the correct option.
Generally, the melting and boiling point of carbon compounds are found to be less than _______ °C.
300
100
200
150
Number of valence electrons in a carbon atom is _______.
4
5
1
3
The bond between two oxygen atoms is _______ bond.
double
triple
single
none of these
The molecular masses of a carbon compound spread over a range of _______.
1012
1014
1010
1013
The unsaturated hydrocarbons containing a carbon-carbon double bond are called _______.
Alkenes
Alkanes
Alkynes
Alcohol
The phenomenon in which compounds having different structural formulae have the same molecular formula is called _______.
structural isomerism
catenation
homologous
functional group
From the following hydrocarbon _______ is the cyclic hydrocarbon.
isobutane
propyne
benzene
isobutylene
While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by _______.
14 u
15 u
16 u
17 u
The general molecular formula for the homologous series of alkynes is _______.
CnH2n
`"C"_"n""H"_("2n" + 2)`
`"C"_"n""H"_("2n" - 2)`
`"C"_"n""H"_("2n" - 1)`
_______ is one of the combustible components of L.P.G.
Methane
Ethane
Propane
Butanol
At room temperature ethanol is _______.
solid
gas
plasma
liquid
Generally _______ is called spirit.
methanol
ethanol
propanol
butanol
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Find the correlation.
Find the correlation.
Solid : iodine : : _______ : bromine
CH3–CH2–CHO : propanal : : CH3–COOH : _______
Ketone : –CO– : : Ester : _______
Cyclohexane : Cyclic hydrocarbon : : Isobutylene : _______
Saturated hydrocarbon : Single bond : : Unsaturated hydrocarbon : _______
Saturated carbon compounds : blue flame : : Unsaturated carbon compounds : _______
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Find odd one out.
Find the odd one out and give its explanation.
Ethylene
Styrene
Propylene
Teflon
Find the odd one out and give its explanation.
Butane
Methane
Benzene
Ozone
Find the odd one out and give its explanation.
CH4
C2H6
C5H12
CaCO3
Find the odd one out and give its explanation.
C2H2
C3H8
C2H6
CH4
Find the odd one out and give its explanation.
C2H4
C4H10
C3H8
CH4
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Answer in one sentence.
Answer the following question in one sentence.
Write the molecular formula of the given compound.
Ethyl ethanoate
Write the molecular formula of the given compound.
Sodium ethanoate
Write the molecular formula of the given compound.
Sodium ethoxide
Write the molecular formula of the given compound.
Stearic acid
Write the molecular formula of the given compound.
Oleic acid
Write the molecular formula of the given compound.
Palmitic acid
Write the molecular formula of the given compound.
Ethylene
Write the molecular formula of the given compound.
Benzene
Write the molecular formula of the given compound.
Acetic acid
Write the molecular formula of the given compound.
Propylene
Write the molecular formula of the given compound.
Acetylene
Write the molecular formula of the given compound.
Ethyl alcohol
Write the molecular formula of the given compound.
Acetone
Write the molecular formula of the given compound.
Propene
Write the molecular formula of the given compound.
Ethanol
Write the molecular formula of the given compound.
Ethanoic acid
Write the molecular formula of the given compound.
Isobutane
Draw electron dot structure and line structure for a given molecule.
Hydrogen
Draw electron dot structure and line structure for a given molecule.
Oxygen
Draw electron dot structure and line structure for a given molecule.
Methane
Draw electron dot structure and line structure for a given molecule.
Nitrogen
Draw electron dot structure and line structure for a given molecule.
Ethene
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Match the columns
Match the pairs.
| Group 'A' | Group 'B' |
| a. C2H6 | 1. Unsaturated hydrocarbon |
| b. C2H2 | 2. Molecular formula of an alcohol |
| c. CH4O | 3. Saturated hydrocarbon |
| d. C3H6 | 4. Triple bond |
Match the columns.
| Group A | Group B |
| 1. Straight chain hydrocarbon | a) Benzene |
| 2. Branched chain hydrocarbon | b) Propyne |
| 3. Cyclic hydrocarbon | c) Isobutylene |
Match the columns.
| Group A | Group B |
| 1) Ether | a) –OH |
| 2) Ketone | b) –O– |
| 3) Ester | c) –CO– |
| 4) Alcohol | d) –COO |
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Right or Wrong sentence
The number of electrons in the valence shell of a carbon atom is 4.
Right
Wrong
Your body is made up of carbon.
Right
Wrong
Carbon compounds contain only open chains of carbon atoms.
Right
Wrong
In general, saturated compounds are more reactive than unsaturated compounds.
Right
Wrong
Benzene is a cyclic unsaturated hydrocarbon.
Right
Wrong
Cyclohexane is a branched chain type of hydrocarbon.
Right
Wrong
As one ascends in any homologous series, physical properties change gradually.
Right
Wrong
There are different general molecular formula for all members of the homologous series.
Right
Wrong
In LPG, butane is a flammable component.
Right
Wrong
Substances that can give oxygen to other substances are called reductant.
Right
Wrong
Potassium permanganate is an oxidizing compound in regular use.
Right
Wrong
Colorless ethanol is in liquid state at room temperature.
Right
Wrong
Ethanol is soluble in water in all proportions.
Right
Wrong
Ester is a sweet-smelling compound.
Right
Wrong
Two carbon atoms can always form one or two covalent bonds.
Right
Wrong
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Write an Explanation
Write an Explanation.
Alkane
Write an Explanation.
Alkene
Write an Explanation.
Alkyne
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Write scientific reasons
Write scientific reason.
Ethylene is an unsaturated hydrocarbon.
Write scientific reason.
The flame appears yellow in the ignition of naphthalene.
Write scientific reason.
The color of iodine disappears in the reaction between vegetable oil and tincture iodine.
Write scientific reason.
Vegetable ghee is formed from the hydrogenation of vegetable oil in presence of nickel catalyst.
Write scientific reason.
Carbon has the property of forming many compounds.
Write scientific reason.
Benzene compounds are called aromatic compounds.
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Solve the following Questions
Explain the Catenation power.
Explain covalent bond with example.
Explain the Structural isomerism term with example.
What is an oxidising agent?
Explain the following term with example.
Reduction
Explain the concept of heteroatoms with the help of examples.
Explain the following reaction with example.
Addition reaction
Explain the following reaction with an example.
Substitution reaction
Explain the following reaction with an example.
Esterification
Explain the following reaction with an example.
Saponification
What are catalysts?
Give a chemical reaction in which a catalyst is used.
Write the characteristics of ethanol.
Write answer as directed.
What is meant by vinegar and gashol? What are their uses?
Write the uses of ethanol.
Write the characteristics of ethanoic acid.
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Distinguish between
Distinguish between:
Saturated hydrocarbons - Unsaturated hydrocarbons
Distinguish between:
Open chain hydrocarbons - Closed chain hydrocarbons
Distinguish between:
Alkane - Alkene
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Write short notes
Write a short note.
Catenation power
Write a short note.
Characteristics of carbon
Write a short note.
Functional groups in carbon compounds
Write a short note.
Homologous series
Write a short note.
Aromatic hydrocarbons
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Answer the Following
Complete the following table for the homologous series of alkanes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methane | CH4 | CH4 | 1 | 1 | -162 |
| Ethane | C2H6 | CH3–CH3 | 2 | 2 | -88.5 |
| Propane | C3H8 | CH3–CH2–CH3 | 3 | 3 | -42 |
| Butane | C4H10 | CH3–CH2–CH2–CH3 | ______ | ______ | 0 |
| Pentane | C5H12 | CH3–CH2–CH2–CH2–CH3 | ______ | ______ | 36 |
| Hexane | C6H14 | CH3–CH2–CH2–CH2–CH2–CH3 | ______ | ______ | 69 |
Complete the following table for homologous series of alcohols.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methanol | CH4O | CH3-OH | 1 | 1 | 63 |
| Ethanol | C2H6O | CH3–CH2-OH | 2 | 2 | 78 |
| Propanol | C3H8O | CH3–CH2–CH2-OH | ______ | ______ | 97 |
| Butanol | C4H10O | CH3–CH2–CH2–CH2–OH | ______ | ______ | 118 |
Complete the following table for homologous series of Alkenes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Ethene | C2H4 | CH2 = CH2 | 2 | 0 | -102 |
| Propene | C3H6 | CH3–CH = CH2 | 3 | 1 | -48 |
| 1-Butene | C4H8 | CH3–CH2–CH = CH2 | ______ | ______ | -6.5 |
| 1-Pentene | C5H10 | CH3–CH2–CH2–CH = CH2 | ______ | ______ | 30 |
Complete the given chart with writing the correct functional group of carbon compounds.
(Ester, Aldehyde, Ketone, Carboxylic acid, Alcohol, Ether)
| -O-H | |
| \[\begin{array}{cc} \ce{O}\phantom{..}\\ ||\phantom{..}\\ \ce{-C-H} \end{array}\] |
|
| \[\begin{array}{cc} \ce{O}\\ ||\\ \ce{-C-} \end{array}\] |
|
| \[\begin{array}{cc} \ce{O}\phantom{.....}\\ ||\phantom{.....}\\ \ce{-C-O-H} \end{array}\] |
|
| -O- | |
| \[\begin{array}{cc} \ce{O}\phantom{..}\\ ||\phantom{..}\\ \ce{-C-O} \end{array}\] |
|
|
\[\begin{array}{cc} |
Complete the following table with writing the correct structural formula and molecular formula.
| Straight chain of carbon atoms | Structural formula | Molecular formula | Name |
| C-C | ______ | ______ | Ethane |
| C-C-C-C | ______ | ______ | Butane |
| C-C-C-C-C-C-C- | ______ | C7H16 | ______ |
| C-C-C-C-C-C-C-C | ______ | C8H18 | ______ |
Complete the following table with writing IUPAC name of carbon compound.
| Sr. No. | Common name | Structural formula | IUPAC Name |
| 1. | ethylene | CH2 = CH2 | |
| 2. | acetylene | HC ≡ CH | |
| 3. | acetic acid | CH3 - COOH | |
| 4. | methyl alcohol | CH3 - OH | |
| 5. | ethyl alcohol | CH3 - CH2 - OH | |
| 6. | acetaldehyde | CH3 - CHO | |
| 7. | acetone | CH3 - CO - CH3 | |
| 8. | ethyl methyl ketone | CH3 - CO - CH2 - CH3 | |
| 9. | ethyl amine | CH3 - CH2 - NH2 | |
| 10. | n-propyl chloride | CH3 - CH2 - CH2 - Cl |
Complete the following activity.
| Boiling point of ethanol | → | |
| General name of ethanol | → | |
| Use of ethanol | → | |
| Boiling point of ethanoic acid | → | |
| Melting point of pure ethanoic acid | → |
Complete the following activity.
Complete the following activity.

Observe the structural formula and answer the following questions.

- Write the name of the given hydrocarbon.
- The given hydrocarbon is included in which type of hydrocarbon?
- What is the kind of compounds with the above characteristic structure called?
Complete the following chart by using examples given in brackets.
(isobutylene, cyclohexane, propane, cyclohexene, cyclopentane, benzene, propyne, isobutane, propene)
| Straight chain hydrocarbons | Branched chain hydrocarbons | Cyclic hydrocarbons |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc}\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\ \phantom{.....}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C = C}\\\phantom{.....}|\phantom{.........}|\\ \phantom{.....}\ce{H}\phantom{........}\ce{H}\end{array}\] |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\ |\phantom{....}|\phantom{....}|\\\ce{H - C - C - C - H}\\ |\phantom{....}|\phantom{....}|\\\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H} \end{array}\] |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc} \phantom{.........}\ce{H}\\ \phantom{.........}|\\ \ce{H - C ≡ C - C - H}\\ \phantom{.........}|\\ \phantom{.........}\ce{H} \end{array}\] |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc} \phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\ \phantom{..}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C- C- H}\\ \phantom{.}|\phantom{....}|\phantom{....}|\\ \ce{H - C - H}\\ |\\\ce{H}\end{array}\] |
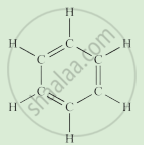
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
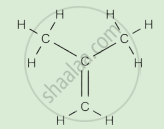
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
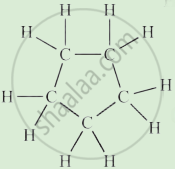
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC 9 Carbon compounds Answer the following questions
Observe the figure and write the answers to the following questions.

- Write the name of the reaction shown in the following figure.
- Write the above chemical reaction in the form of a balanced equation.
- Write the name of the product produced in the above reaction, write a use.
- Write the name of the catalyst used in the above reaction.
Write the answers to the questions by observing the following figure.

- Write the chemical reaction shown in the figure above in the form of a balanced equation.
- Write the name of the gas coming out of the large test tube in the above chemical reaction.
- Why do small bubbles appear in the small test tube?
- What is the change in colour of lime water?
Solutions for 9: Carbon compounds
![SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC chapter 9 - Carbon compounds SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC chapter 9 - Carbon compounds - Shaalaa.com](/images/science-and-technology-1-english-10-standard-ssc_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
SCERT Maharashtra solutions for Science and Technology 1 [English] 10 Standard SSC chapter 9 - Carbon compounds
Shaalaa.com has the Maharashtra State Board Mathematics Science and Technology 1 [English] 10 Standard SSC Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. SCERT Maharashtra solutions for Mathematics Science and Technology 1 [English] 10 Standard SSC Maharashtra State Board 9 (Carbon compounds) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. SCERT Maharashtra textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Science and Technology 1 [English] 10 Standard SSC chapter 9 Carbon compounds are Functional Groups in Carbon Compounds, Homologous Series of Carbon Compound, Ethanol, Ethanoic Acid, Hydrocarbons, Macromolecules and Polymers, Bonds in Carbon Compounds, Carbon: a Versatile Element, Properties of Carbon, The IUPAC System of Nomenclature, Carbon Compounds in Everyday Life, Nomenclature of Organic Compounds, Chemical Properties of Carbon Compounds, Structural Variations of Carbon Chains in Hydrocarbons.
Using SCERT Maharashtra Science and Technology 1 [English] 10 Standard SSC solutions Carbon compounds exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in SCERT Maharashtra Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Science and Technology 1 [English] 10 Standard SSC students prefer SCERT Maharashtra Textbook Solutions to score more in exams.
Get the free view of Chapter 9, Carbon compounds Science and Technology 1 [English] 10 Standard SSC additional questions for Mathematics Science and Technology 1 [English] 10 Standard SSC Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
