Advertisements
Advertisements
Question
There are different general molecular formula for all members of the homologous series.
Options
Right
Wrong
Solution
There are different general molecular formula for all members of the homologous series- Wrong
APPEARS IN
RELATED QUESTIONS
Write the next homologue of the following: C2H4
Write the name and formula of the 2nd member of homologous series having general formula CnH2n – 2.
Fill in the following blank with suitable word:
Ethene and ethyne are examples of ..... hydrocarbons.
Fill in the following blank with suitable word:
Carbon compounds have usually ... melting points and boiling points because they are ...... in nature.
What is the next higher homologue of methanol (CH3OH)?
Give the molecular formula of one homologue of the following:
C3H6
Give the molecular formula of one homologue of each of the following:
C2H6
By how many carbon atoms and hydrogen atoms do any two adjacent homologues differ?
Write the molecular formula of the third member of the homologous series of carbon compounds with general formula CnHO2n+1OH.
The number of carbon atoms present in the molecule of fifth member of the homologous series of alkynes is:
(a) four
(b) five
(c) six
(d) seven
Give the dot diagram of the first member of the alcohol.
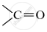 .
.
Study the different conclusions drawn by students of a class on the basis of observations of preserved/available specimens of plants and animals.
I. Potato and sweet potato are analogous organs in plants.
II. Wings of insects and wings of birds are homologous organs in animals.
III. Wings of insects and wings of bats are analogous organs in animals.
IV. Thorns of citrus and tendrils of cucurbita are analogous organs in plants.
The correct conclusions are:
(A) I, and II
(B) II and IV
(C) I and III
(D) III and IV
Copy and complete the following table which relates to three homologus series of hydrocarbons:
| General formula | CnH2n | CnH2n-2 | CnH2n+2 |
| IUPAC name of the homologus series | |||
| Characteristic bond type | Single bonds | ||
| IUPAC name of the first member of the series | |||
| Type of reaction with chlorine | Addition |
What is the difference in the molecular formula of any two adjacent homologues:
(i) In terms of molecular mass
(ii) In terms of number and kind of atoms per molecule?
While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by _______.
As one ascends in any homologous series, physical properties change gradually.
Consider the carbon compounds having following molecular formula:
(i) C3H6 (ii) C3H8 (iii) C4H6 (iv) C6H6 (v) C6H12
- State the number of double covalent bonds present in C3H8.
- Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
- Which one of the above compounds forms a ring structure of carbon atoms?
- Identify, which of the above compounds, is a member of alkane series.
